The aim of the present study is to examine the possible effect de dexmedetomidine on the development of morphine tolerance in rats including nociception, morphine analgesia, apoptosis, oxidative stress, and tumour necrosis factor (TNF)/ interleukin-1 (IL-1) pathways.
Materials and methodsIn this study, 36 Wistar Albino (225–245 g) rats were used. Animals were divided into 6 groups: saline (S), 20 mcg/kg dexmedetomidine (D), 5 mg/kg morphine (M), M + D, morphine tolerance (MT), and MT + D. The analgesic effect was measured with hot plate and tail-flick analgesia tests. After the analgesia tests, the dorsal root ganglia (DRG) tissues were excised. Oxidative stress parameters [total antioxidant status (TAS), total oxidant status (TOS)], TNF, IL-1 and apoptosis enzymes (Caspase-3, Caspase-9), were measured in DRG tissues.
ResultsDexmedetomidine showed an antinociceptive effect when given alone (p < 0.05 to p < 0.001). In addition, dexmedetomidine increased the analgesic effect of morphine (p < 0.001), and also decreased the tolerance to morphine at a significant level (p < 0.01 to p < 0.001). Moreover, it decreased oxidative stress (p < 0.001) and TNF/IL-1 levels when given as an additional drug of single-dose morphine and morphine tolerance group (p < 0.001). Furthermore, dexmedetomidine decreased Caspase-3 and Caspase-9 levels after tolerance development (p < 0.001).
ConclusionDexmedetomidine has antinociceptive properties, and it increases the analgesic effect of morphine and also prevents tolerance development. These effects probably occur by the modulation of oxidative stress, inflammation and apoptosis.
El objetivo del presente estudio es examinar el posible efecto de dexmedetomidina en el desarrollo de tolerancia a la morfina en ratas, incluyendo nocicepción, analgesia con morfina, apoptosis, estrés oxidativo, y las vías del factor de necrosis tumoral (TNF)/ interleucina-1 (IL-1).
Materiales y métodosEn este estudio, se utilizaron 36 ratas Wistar Albino (225–245 g) dividiéndose a los animales en 6 grupos: solución salina (S), 20 mcg/kg de dexmedetomidina (D), 5 mg/kg de morfina (M), M + D, tolerancia a la morfina (MT), y MT + D. El efecto analgésico se midió mediante las pruebas analgésicas de placa caliente (hot plate) y de retirada de la cola (tail flick). Tras dichas pruebas, se extirparon los ganglios de la raíz dorsal (GRD), y se midieron en los tejidos de los mismos los parámetros del estrés oxidativo [estado antioxidante total (TAS), estado oxidante total (TOS)], TNF, IL-1 y enzimas de la apoptosis (Caspasa-3, Caspasa-9).
ResultadosDexmedetomidina reflejó un efecto antinociceptivo al administrarse en solitario (p < 0,05 a p < 0,001). Además, dexmedetomidina incrementó el efecto analgésico de la morfina (p < 0,001), y también redujo la tolerancia a la morfina a un nivel significativo (p < 0,01 a p < 0,001), reduciendo también los niveles de estrés oxidativo (p < 0,001) y TNF/IL-1 al administrarse como fármaco adicional al grupo de dosis única de morfina y tolerancia a la morfina (p < 0,001). Además, dexmedetomidina redujo los niveles de Caspasa-3 y Caspasa-9 tras el desarrollo de tolerancia (p < 0,001).
ConclusiónDexmedetomidina tiene propiedades antinociceptivas, e incrementa el efecto analgésico de la morfina, previniendo también el desarrollo de tolerancia. Estos efectos se producen probablemente debido a la modulación del estrés oxidativo, la inflamación y la apoptosis.
Morphine is a μ-opiate receptor agonist frequently used in the treatment of chronic and severe pain due to its analgesic effect, but its efficacy decreases over time due to tolerance to its antinociceptive properties. Although many studies have been conducted to investigate opioid tolerance, the cause is still unknown.1 Recent studies suggest that proinflammatory cytokines, especially interleukin (IL)-1β, play a role in the development of morphine tolerance.2–4
Glia cells increase sensitivity to pain by stimulating secretion of proinflammatory cytokines, such as TNF and IL-1,5,6 and some studies have reported that hyperalgesia develops with exogenous IL-1 administration7; therefore, IL-1 receptor antagonist (IL-1ra) treatment reduces inflammation-related hyperalgesia.8,9 Moreover, anakinra, an IL-1 antagonist, was found to reduce morphine tolerance,10 and the TNF-α antagonist etanercept also preserves the antinociceptive effect of morphine.11 In the light of these findings, it is safe to say that pro-inflammatory cytokines such as IL-1 and TNF-α modulate pain sensitivity in basal and inflammatory conditions.
Dexmedetomidine, a selective α2 adrenergic agonist, may cause hypnosis, sedation, analgesia, and minimal respiratory depression.12 Its most frequent adverse effects are hypotension and bradycardia.13 In recent years, the effect of dexmedetomidine on the inflammatory process has also been studied. In a meta-analysis of 15 randomized controlled studies, the authors concluded that dexmedetomidine reduces TNF-α, IL-6, and IL-8 secretions, and called for more studies on its effect on pro-inflammatory cytokines, including IL-1.14 A recent review of the literature showed the neuroprotective effect of dexmedetomidine, its restrictive effect on neuroinflammation, and its inhibitive effect on apoptosis and oxidative stress.15
Peroxynitrite (ONOO−), derived from its precursors superoxide and nitric oxide (NO), plays an important role in morphine-mediated cell processes. Neuronal NO is generated from l-arginine and molecular oxygen by Ca2+/calmodulin-activated NO synthase (nNOS) in response to Ca2+ u mediated by the NMDA receptor.16 O2− is primarily generated by disruption of the mitochondrial electron transport chain, and is a product of cytosolicoxidases such as NADPH oxidase and xanthineoxase.17 nNOS is also capable of producing O2− in addition to NO under low-arginine conditions, constituting an ‘ONOO−-synthase’ activity.18 Although opioid tolerance is clearly not a monolithic phenomenon, there are substantial data showing that overproduction of peroxynitrite (ONOO-) under neuroinflammatory conditions plays a key role. ONOO- is a potent proapoptotic species, and spinal neuronal apoptosis also plays a role in morphine antinociceptive tolerance.19,20 All these oxidative molecules are involved in the development of pain, opioid-induced hyperalgesia, and opioid tolerance.21
The aim of this study is to examine the possible effect of dexmedetomidine on the development of morphine tolerance in rats, including nociception, morphine analgesia, apoptosis, oxidative stress, and IL-1/TNF pathways. We believe that the results of this study will contribute greatly to our understanding of the developmental mechanism of morphine tolerance and the effect of dexmedetomidine on this phenomenon.
Materials and methodsAnimalsWistar Albino rats (18 male, 18 female, 6 groups, 225−245 g per group; n = 6; 36 rats in total) were obtained from the Cumhuriyet University Animal Laboratory and housed under standard conditions. Rats were equally divided into groups in terms of gender. Standard conditions in the rats' environment were: 12 h of light and dark (lights on at 07:00 am) and constant temperature (22 ± 2 °C) with ad libitum food and water. All the experiments were performed between 08:00 and 16:00 h. Rats were prepared for all procedures, which were performed following the guidelines of the National Institute of Health: “Animal Laboratory Care Principles”. The study was approved by the Sivas Cumhuriyet University Animal Ethics Committee (No: 65202830-050.04.04-435).
DrugsDexmedetomidine (Dextomid, 200 mcg/2 ml iv) and morphine sulphate were dissolved in saline solution. The drugs were dissolved freshly on the days of intervention. Before analgesia tests, dexmedetomidine (20 mcg/kg) and saline were administered intraperitoneally (ip) and morphine (5 mg/kg) subcutaneously (sc).
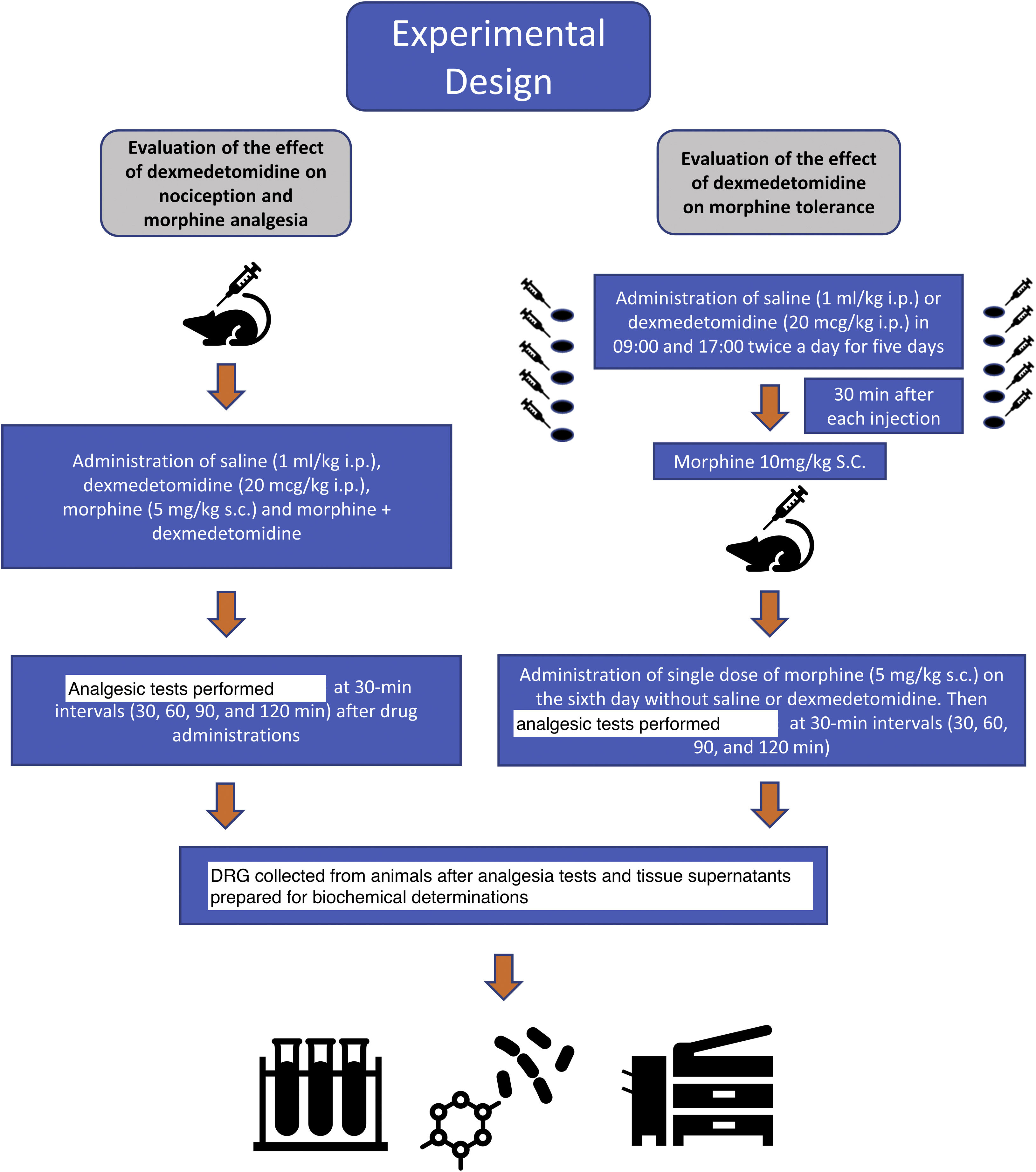
Experimental protocolsThe analgesic effects of dexmedetomidine and morphine were evaluated at 30-minute intervals (30, 60, 90 and 120 min) using hot-plate and tail-flick antinociception tests. The animals were divided into 6 groups: group S: saline, group D: dexmedetomidine 20 mcg/kg, group M: morphine 5 mg/kg, group M + D: morphine + dexmedetomidine, group MT: morphine tolerance, and group MT + D: morphine tolerance + dexmedetomidine. The animals were sacrificed by decapitation with no anaesthetic or analgesic agent after analgesic tests were performed. Tissue samples of the dorsal root ganglia (DRG) at the level of T12-L5 were obtained from all animals for evaluation (Fig. 1).
Antinociception testsTail-flick testA standard medical device (May TF 0703 Tail-flick Unit, Commat, Turkey) was used to measure thermal nociception during the tail-flick test. A heat source was focused 3 cm away from the distal part of the tail for each measurement after administration of the study drugs or saline solution. Tail-flick latencies (TFL) were measured once the saline or the drugs had been administered. The cut-off latency time was set to 15 s to avoid tissue injury. Central pain mechanisms were the underlying cause of the hyperalgesic response in the tail withdrawal test.
Hot-plate testThe antinociceptive reaction to the hot-plate is associated with both central and peripheral mechanisms. Rats were individually placed on a hot plate (May AHP 0603 Analgesic Hot Plate, Commat, Turkey) at 54 ± 2 °C. Latency until the first paw-licking or jumping reaction was recorded as an indicator of pain threshold, in order to avoid the high-temperature stimulus. The cut-off time was 30 s to avoid tissue damage to paws.
Morphine tolerance inductionThe rats were selected randomly and treated with 10 mg/kg morphine sc twice a day (at 08:00 and at 16:00 h) for 5 days to induce morphine tolerance. Morphine (10 mg/kg) was administered for 30 min after each dexmedetomidine injection for 5 days to determine the effects of dexmedetomidine (20 mcg/kg, ip) on morphine tolerance in group MT + D. On day 6, 5 mg/kg sc morphine was administered without saline or dexmedetomidine to provide optimal analgesia. Then, tail-flick and hot-plate tests were repeated at 30-minute intervals (30, 60, 90 and 120 min) to assess the degree of morphine tolerance.
DRG tissue homogenate preparationDRG tissue samples were homogenated in cold phosphate buffered saline solution in a homogenizer (Speed Mill Plus, Analytik Jena, Germany) and then centrifuged at 4000 rpm at 4 °C for 10 min. All the supernatants were obtained and stored at −80 °C for 72 h until biochemical analysis. Total protein levels of the samples were detected using a Bradford protein assay kit (Merck, Germany).22
Total antioxidant status (TAS) measurementTAS concentrations in tissue were determined using an automated assay method previously developed by Erel based on monitoring the reaction rate of free radicals released during the Fenton reaction.23 In this method, antioxidants in tissue samples suppress coloration in proportion to their concentration. The results obtained were expressed as micromolar Trolox equivalents per gram tissue protein (μmol Trolox Eq/g protein).
Total oxidant status (TOS) measurementTOS concentrations in tissue level were measured with Erel’s automated assay method.18 In this method, TOS levels are measured by using xylenol orange to determine the levels of ferric ions in tissue. The assay is calibrated with hydrogen peroxide.24 The outcomes of the assay were expressed as micromolar hydrogen peroxide equivalents per gram tissue protein (μmol H2 O2 Eq/g protein).
Measurement of IL-1, TNF, Caspase-3, and Caspase-9Rat ELISA kits (Shanghai Sunred Biological Technology, Shanghai, China) were used to determine levels IL-1, TNF, Caspase-3, and Caspase-9 in DRG supernatants. Standard and tissue samples were plated and incubated for 60 min at 37 °C. After washing the plates, staining solutions were added and the plates were incubated for 15 min at 37 °C. Then, stop solution was added and read at 450 nm. Standard curves were used to calculate all kits. The intra- and inter-plate variation coefficients were under 10%.
Analgesic data analysisTo calculate the percentage of maximum antinociceptive effect (% MPE), tail-flicks and hot-plate latencies (measured in seconds) were converted to an antinociceptive effectiveness percentage using the following equation: % MPE = ([post drug latency – baseline latency)/(cut-off value – baseline latency]) × 100.
StatisticsThe results are shown as mean ± SEM (standard error of the mean). The antinociceptive effect was measured and the mean % MPEs were calculated. Analysis of variance (one-way anova) and a posthoc Tukey test were used to analyse the data. The significance value was set as p < 0.05.
ResultsThe gender of the rats did not influence the results (p > 0.05).
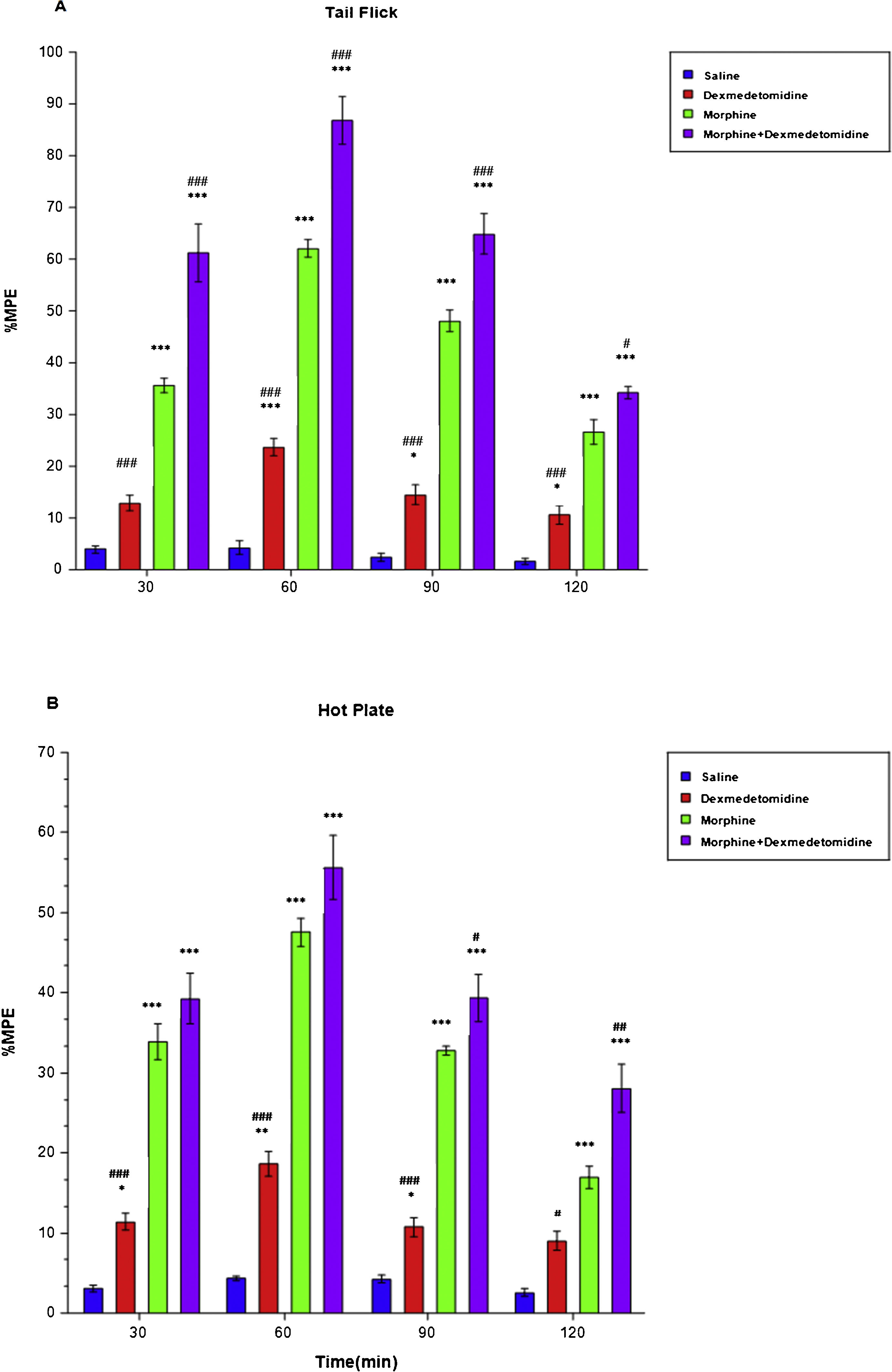
Effect of dexmedetomidine on nociception and morphine analgesiaAnalgesic responses were determined with analgesic tests following administration of 20 mcg/kg dexmedetomidine at 30-min intervals for 2 h in order to evaluate the effect of dexmedetomidine on nociception and morphine analgesia. When the antinociception in the dexmedetomidine group (23.62 ± 1.70, LCL [lower confidence limit]: 19.25, UCL [upper confidence limit]: 27.99) was compared with the saline group (4.29 ± 1.22, LCL: 1.16, UCL: 7.41), a significant difference was found in tail-flick tests at minute 60 (p < 0.001), 90 (p < 0.05) (D: 14.47 ± 1.94, LCL: 9.49, UCL: 19.45, S: 2.39 ± 0.71, LCL: 0.56, UCL: 4.22) and 120 (p < 0.05) (D: 10.57 ± 1.82, LCL: 5.90, UCL: 15.23, S: 1.63 ± 0.64, LCL: −0.01, UCL: 3.27) (Fig. 2A). In hot plate tests, when antinociception in the dexmedetomidine and saline groups were compared, a significant difference was found at minute 30 (D: 11.35 ± 1.05, LCL: 8.66, UCL: 14.05 S: 3.07 ± 0.41, LCL: 2.00, UCL: 4.13), 90 (D: 10.77 ± 1.18, LCL: 7.77, UCL: 13.81, S: 4.26 ± 0.44, LCL: 3.13, UCL: 5.40), (p < 0.05) and 60 (D: 18.62 ± 1.59, LCL: 14.54, UCL: 22.71, S: 4.33 ± 0.30, LCL: 3.55, UCL: 5.11), (p < 0.01) (Fig. 2B). However, after administration of 20 mcg/kg dexmedetomidine, the maximum analgesic effect was observed at minute 60 in both tail-flick and hot-plate tests.
These findings show that the antinociceptive effect of dexmedetomidine + morphine significantly increased in the tail-flick test (p < 0.001) at minute 30 (M: 35.64 ± 1.43, LCL: 31.96, UCL: 39.33, M + D: 61.18 ± 5.52, LCL: 46.97, UCL: 75.38), (p < 0.001), 60 (M: 62.05 ± 1.68, LCL: 57.74, UCL: 66.36, M + D: 86.71 ± 4.60, LCL: 74.89, UCL: 98.52), (p < 0.001), 90 (M: 48.03 ± 2.10, LCL: 42.64, UCL: 53.42, M + D: 64.88 ± 3.82, LCL: 55.05, UCL: 74.71), (p < 0.001), and 120 (M: 26.60 ± 2.35, LCL: 20.56, UCL: 32.65, M + D: 34.24 ± 1.15, LCL: 31.29, UCL: 37.19), (p < 0.05) (Fig. 2A). In the hot-plate tests, at minute 90 (M: 32.79 ± 0.55, LCL: 31.39, UCL: 34.19, M + D: 39.35 ± 2.93, LCL: 31.82, UCL: 46.88), (p < 0.05) and 120 (M: 16.93 ± 1.42, LCL: 13.29, UCL: 20.57, M + D: 28.07 ± 3.06, LCL: 20.21, UCL: 35.93), (p < 0.01) dexmedetomidine significantly increased the antinociceptive effect of morphine (Fig. 2B). Additionally, the peak dexmedetomidine + morphine analgesic effect was achieved at minute 60 in the tail-flick (86.71 ± 4.60, LCL: 74.89, UCL: 98.52) and hot-plate (55.60 ± 3.99, LCL: 45.35, UCL: 65.86) tests.
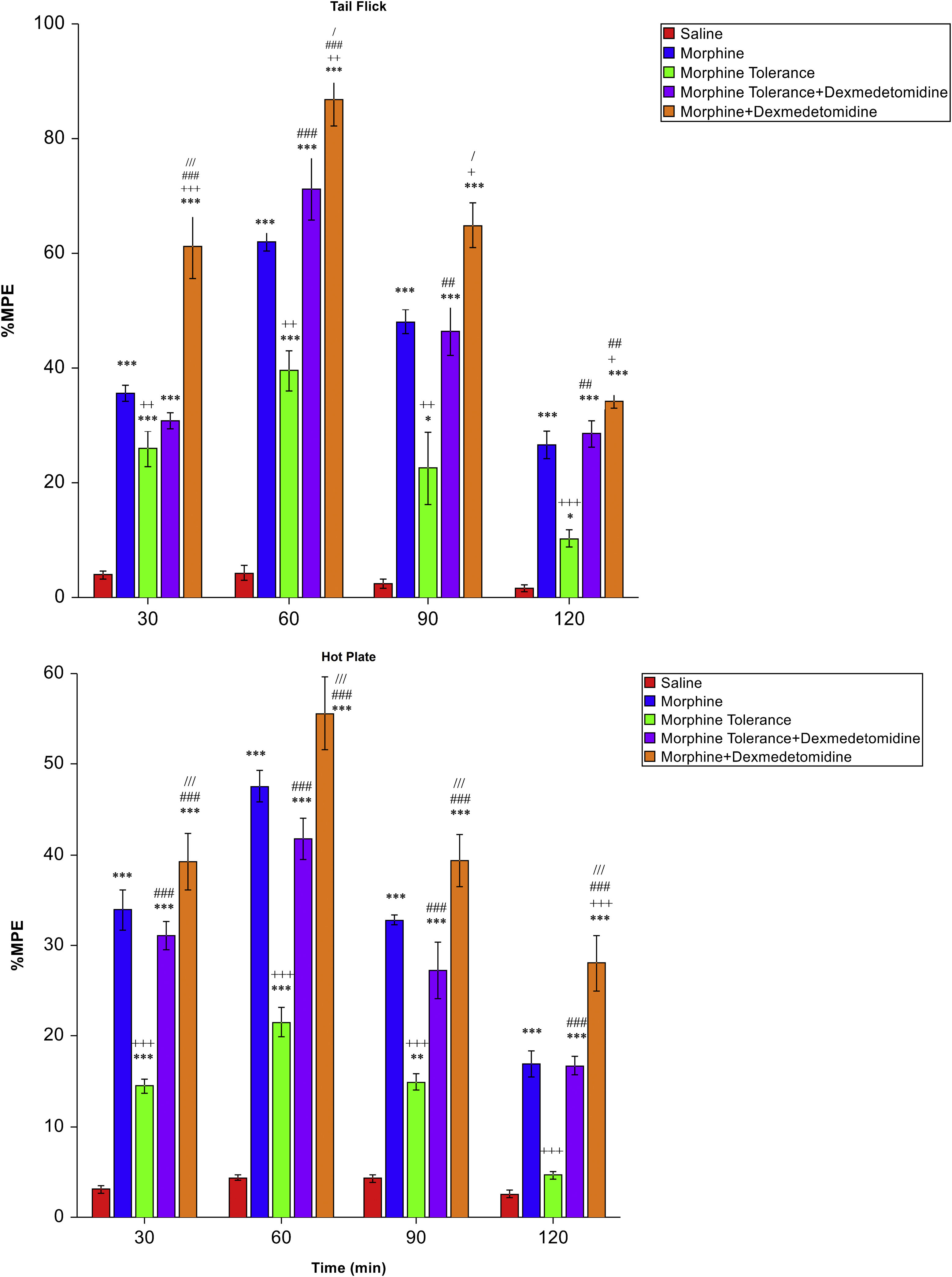
Effect of dexmedetomidine on morphine toleranceMPE% values of the morphine group were statistically and significantly higher than in the morphine tolerance group in both the tail-flick (p < 0.01 to p < 0.001; Fig. 3A) and hot-plate (p < 0.001; Fig. 3B) tests. Dexmedetomidine significantly reduced morphine tolerance in the tail-flick test at minute 60 (MT: 39.54 ± 3.53, LCL: 30.46, UCL: 48.62, MT + D: 71.28 ± 5.46, LCL: 57.24, UCL: 85.32), 90 (MT: 22.58 ± 6.31, LCL: 6.36, UCL: 38.79, MT + D: 46.48 ± 4.38, LCL: 35.22, UCL: 57.73) and 120 (MT: 10.27 ± 1.47, LCL: 6.51, UCL: 14.04, MT + D: 28.59 ± 2.29, LCL: 22.70, UCL: 34.47) (p < 0.01 to p < 0.001; Fig. 3A) and in all measurement times in the hot-plate test (p < 0.001; Fig. 3B).
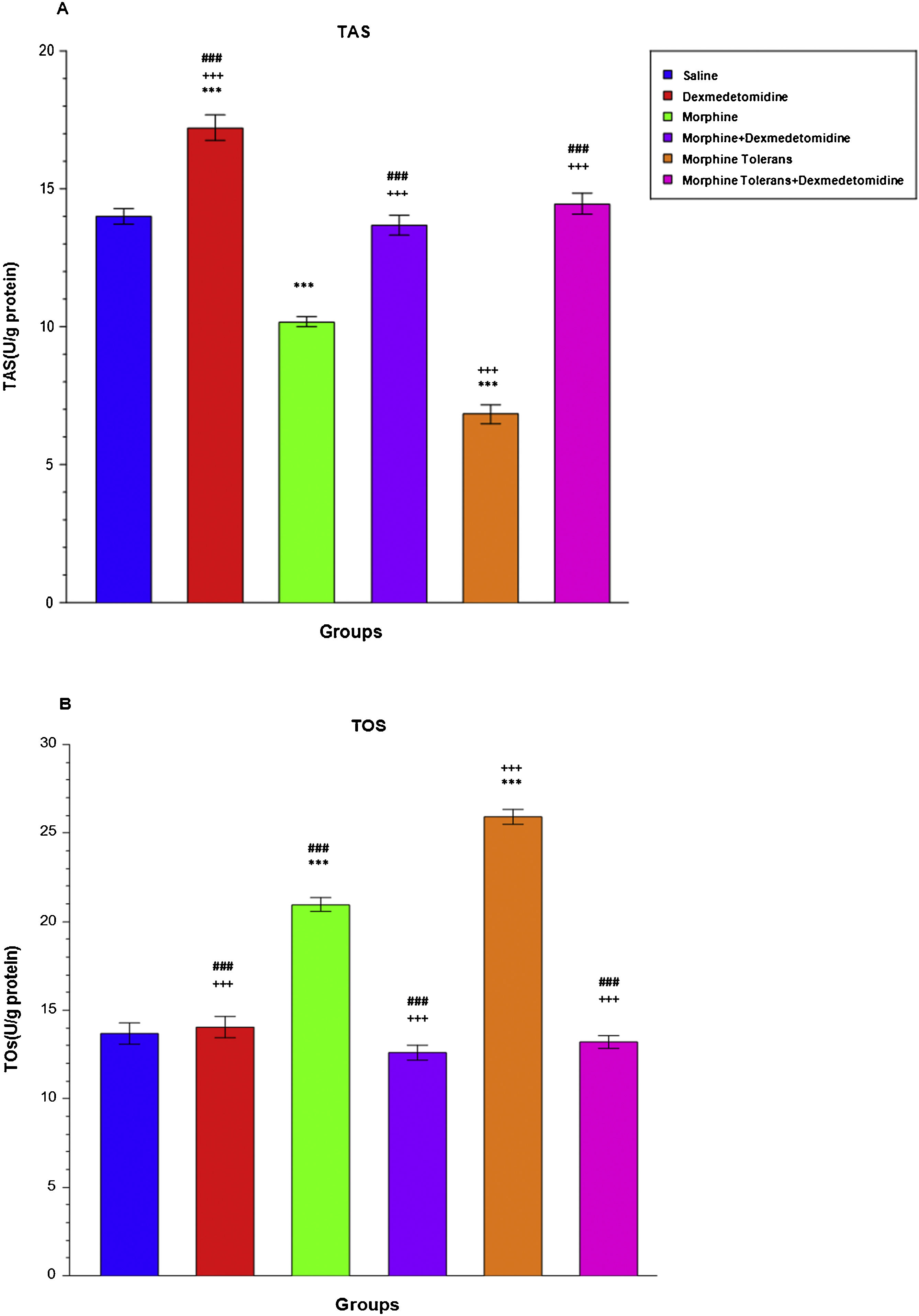
Effect of dexmedetomidine on antioxidant and oxidant parameters (TAS and TOS levels) in morphine analgesia and tolerance in DRGA single dose of morphine and the development of morphine tolerance significantly decreased TAS levels in DRGs compared to the saline group (M: 10.17 ± 0.18, LCL: 9.71, UCL: 10.63, MT: 6.84 ± 0.34, LCL: 5.97, UCL: 7.71, S: 14.01 ± 0.28, LCL: 13.30, UCL: 14.72), (p < 0.001; Fig. 4A). The addition of dexmedetomidine significantly increased TAS levels compared to the morphine and morphine tolerance group (M + D: 13.68 ± 0.36, LCL: 12.75, UCL: 14.61, MT + D: 14.46 ± 0.38, LCL: 13.48, UCL: 15.44), (p < 0.001; Fig. 4A). A single injection of morphine significantly increased TOS levels compared to the saline group (M: 20.97 ± 0.41, LCL: 19.92, UCL: 20.02, S: 13.66 ± 0.60, LCL: 12.11, UCL: 15.21) (p < 0.001; Fig. 4B). When dexmedetomidine was combined with morphine, a significant decrease was observed in TOS levels compared to the single-dose morphine group (M + D: 12.59 ± 0.41, LCL: 11.54, UCL: 13.64, M: 20.97 ± 0.41, LCL: 19.92, UCL: 20.02) (p < 0.001; Fig. 4B). Morphine tolerance significantly increased TOS levels in DRGs compared to both the saline group and the morphine group (MT: 25.92 ± 0.42, LCL: 24.84, UCL: 26.99, S: 13.66 ± 0.60, LCL: 12.11, UCL: 15.21, M: 20.97 ± 0.41, LCL: 19.92, UCL: 20.02), (p < 0.001; Fig. 4B). The addition of dexmedetomidine to morphine tolerance significantly decreased TOS levels (MT: 25.92 ± 0.42, LCL: 24.84, UCL: 26.99, MT + D: 13.21 ± 0.35, LCL: 12.31, UCL: 14.11), (p < 0.001; Fig. 4B).
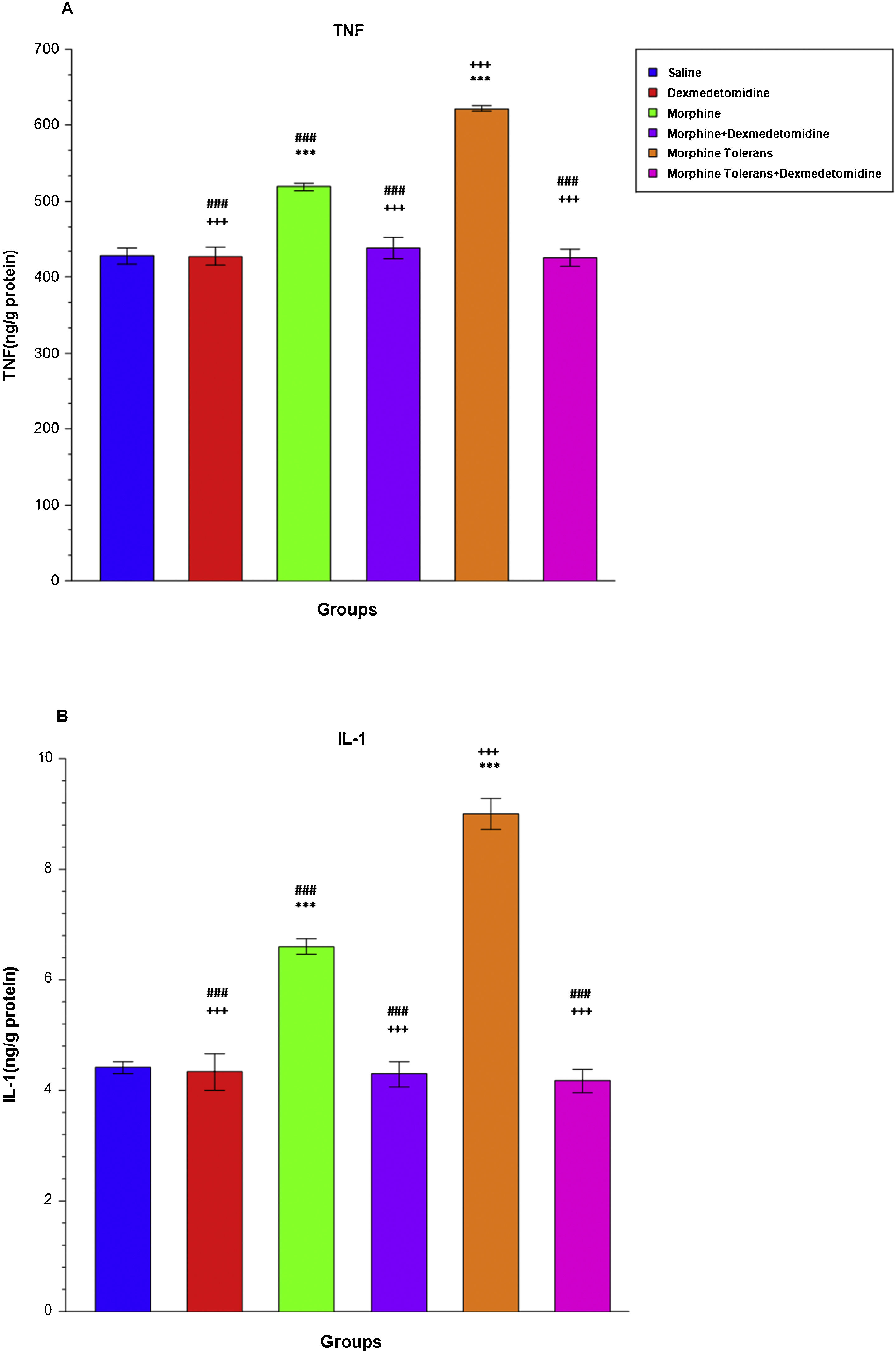
Effect of dexmedetomidine on antioxidant and oxidant parameters (TAS and TOS levels) in morphine analgesia and tolerance in DRG. (a) shows the effect of dexmedetomidine on TAS levels in morphine analgesia and tolerance in DRG; (b) shows the effect of dexmedetomidine on TOS levels in morphine analgesia and tolerance in DRG.
A single dose of morphine significantly increased TNF (M: 518.92 ± 5.20, LCL: 505.55, UCL: 532.30, S: 427.77 ± 10.17, LCL: 401.61, UCL: 453.92) and IL-1 levels (M: 6.60 ± 0.14, LCL: 6.24, UCL: 6.96, S: 4.41 ± 0.11, LCL: 4.12, UCL: 4.71) compared to the saline group (p < 0.001; Fig. 5A and B). Dexmedetomidine combined with single-dose morphine significantly decreased TNF (M + D: 437.92 ± 14.17, LCL: 401.50, UCL: 474.33) and IL-1 levels (M + D: 4.29 ± 0.23 LCL: 3.69, UCL: 4.90) compared to the morphine group (p < 0.001; Fig. 5A and B). When the morphine tolerance group was compared to the single-dose morphine group, a statistically significant increase was observed in TNF (MT: 621.90 ± 3.33, LCL: 613.34, UCL: 630.45) and IL-1 levels (MT: 9.00 ± 0.29, LCL: 8.26, UCL: 9.73), (p < 0.001; Fig. 5A-B). The addition of dexmedetomidine to morphine tolerance significantly decreased TNF (MT + D: 425.78 ± 11.40, LCL: 396.48, UCL: 455.07) and IL-1 levels (MT + D: 4.17 ± 0.21, LCL: 3.64, UCL: 4.70), (p < 0.001; Fig. 5A and B).
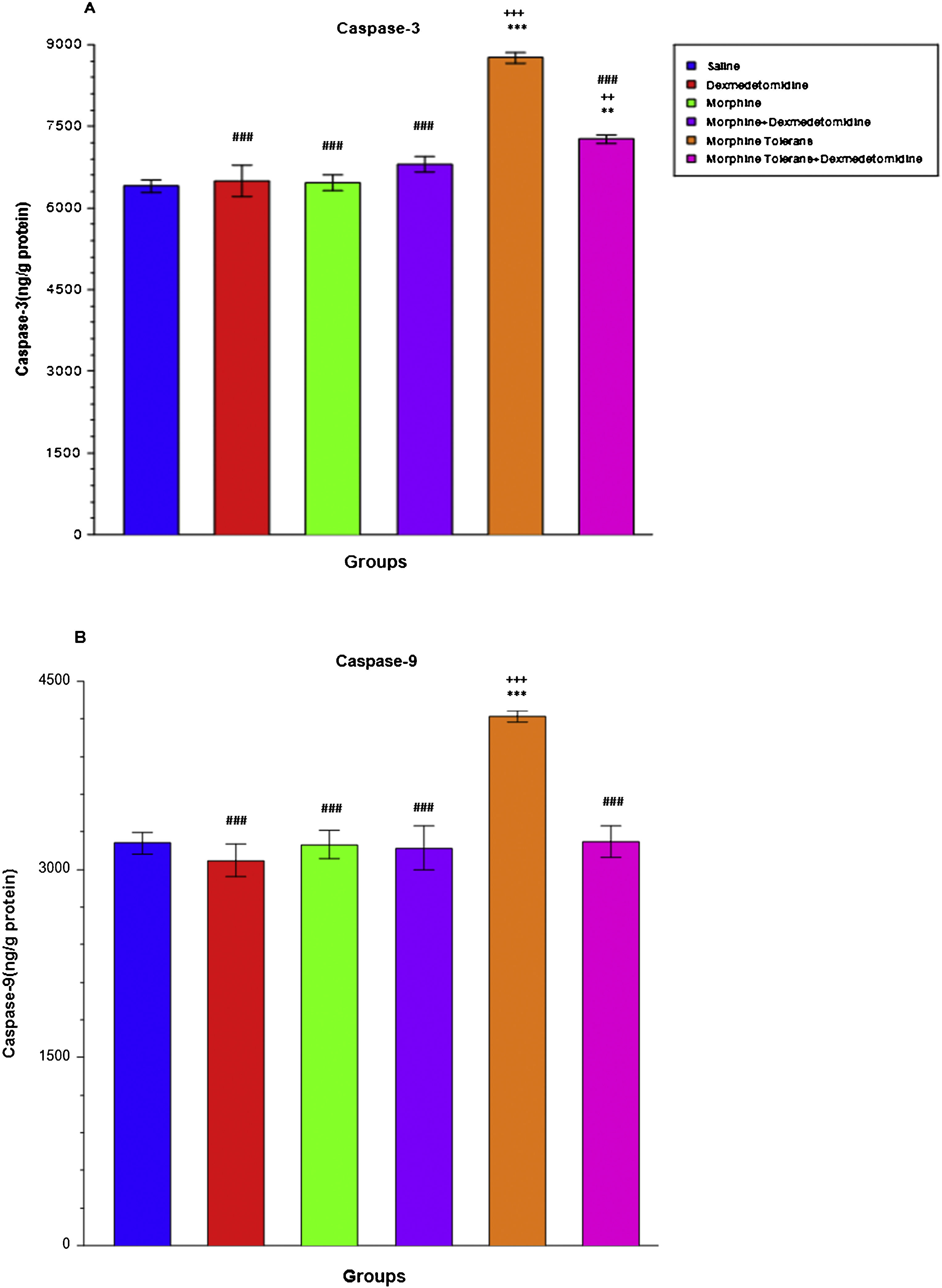
Effect of dexmedetomidine on apoptosis (Caspase-3 and Caspase-9) in morphine analgesia and tolerance in DRA single-dose administration of morphine did not statically alter Caspase-3 (M: 6456.77 ± 144.32, LCL: 6085.79, UCL: 6827.74, S: 6403.61 ± 113.27, LCL: 6112.44, UCL: 6694.78) and Caspase-9 levels (M: 3198.78 ± 109.59, LCL: 2917.06, UCL: 3480.50, S: 3209.91 ± 87.77, LCL: 2984.28, UCL: 3435.53) in DRGs compared to the saline group. The morphine tolerance group showed significantly increased Caspase-3 (MT: 8761.72 ± 102.11, LCL: 8499.23, UCL: 9024.21) and Caspase-9 levels (MT: 4220.68 ± 41.83, LCL: 4113.15, UCL: 4328.21) compared to the single-dose morphine and saline groups (p < 0.001; Fig. 6A and B). When dexmedetomidine was added to morphine tolerance, a significant decrease was observed in Caspase-3 (MT + D: 7268.64 ± 81.39, LCL: 7059.41, UCL: 7477.87) and Caspase-9 levels (MT + D: 3220.02 ± 124.10, LCL: 2901.01, UCL: 3539.03) compared to the morphine tolerance group (p < 0.001; Fig. 6A and B).
DiscussionMicroinjection of morphine in the ventrolateral periaqueductal gray region is known to inhibit the nociceptive tail response through acetylcholine, 5-HT and α2 receptors in the spinal cord.25 In our study on the effect of dexmedetomidine (an α2 agonist with sedative, anxiolytic, and analgesic properties) on morphine analgesia, morphine tolerance, inflammation, and apoptosis, we conducted tail-flick and hot-plate tests to show that dexmedetomidine increased morphine analgesia and inhibited morphine tolerance development by decreasing pro-inflammatory cytokines (TNF and IL-1 levels) and the apoptotic enzymes (Caspase-3 and Caspase-9 levels).
After performing a literature search, we found only 1 study that analysed the relationship between dexmedetomidine and morphine tolerance. Gursoy et al. developed morphine tolerance in rats by administering 50 mg/kg/day morphine for 3 days, and found that dexmedetomidine reduced morphine tolerance in tail-flick and hot-plate tests.26 Although we used different morphine doses, we reached the same conclusion after performing the tail-flick and hot-plate tests. However, unlike Gursoy et al., we also evaluated to apoptotic and anti-inflammatory properties of dexmedetomidine.
In healthy volunteers, dexmedetomidine exhibits rapid distribution. Non-compartmental analysis has shown it to have a distribution half-life (t1/2α) of about 6 min. The mean terminal elimination half-life (t1/2) is approximately 2.1–3.1 h.27 Karna et al. administered 10 mcg/kg iv dexmedetomidine in rats, and measured the peak analgesic effect at 45 min in the hot-plate test and at 60 min in tail-flick test; the analgesic effect of dexmedetomidine was reduced at 120 min in tail-flick test. Our doses and routes of administration of dexmedetomidine differed from those used in Karna et al. In rats, duration of analgesia can vary when the drug is administered as a single-shot rather than in infusion.28
Dexmedetomidine was administered at a dose of 10 mcg/kg and 20 mcg/kg in most animal studies in literature.26,28 We, however, administered 20 mcg/kg rather than 10 mcg/kg in order to assess apoptosis and the anti-inflammatory effect.
Dexmedetomidine is known to reduce the release of inflammatory cytokines such as TNF-α and IL-1 through the α2 adrenergic receptor.29 Wang et al., in 2017, showed that dexmedetomidine attenuated the inflammatory response by inhibiting nuclear factor-kappa B (NF-kB) activation in ischaemia/reperfusion injury.29 Previous studies have reported that dexmedetomidine provides analgesia by attenuating the inflammation that causes neuropathic pain.30 According to Kim et al., in 2017, dexmedetomidine inhibits inflammation by deactivating Toll-like receptor-4 (TLR4) and NF-kB pathway in cerebral ischaemia/reperfusion injury, and thus protects neurons after cerebral ischaemia.31 Our study investigated the adverse effects of single-dose morphine and morphine tolerance in healthy rats, and we found that one such adverse effect was inflammation secondary to increased TNF and IL-1 levels in single-dose morphine and morphine tolerance. Several studies have shown that morphine stimulates the release of inflammatory cytokines10,32; and in our study, we found that dexmedetomidine reduced the high levels of TNF and IL-1 caused by single-dose morphine and morphine tolerance.
We observed that the combination of morphine and dexmedetomidine enhanced the analgesic effect. Wei et al. recently showed that acid-sensing ion channels (ASICs), which are prevalent in DRGs, play an important role in tissue acidosis-induced pain.33 We also examined DRGs, and our results suggest that that dexmedetomidine-induced ASIC blockade may be responsible for the enhanced analgesic effect of morphine + dexmedetomidine compared to morphine alone.
There is a growing body of evidence that morphine not only activates the relevant receptors and their traditional signalling pathways, but can also elicit oxidative stress under certain conditions. Importantly, oxidative stress appears to play a significant role in the development of different pathological processes. On this basis, it is reasonable to assume that formation of reactive oxygen (ROS) or reactive nitrogen (RNS) species may also underlie some of the adverse physiological effects of morphine.34 Morphine has received far more attention than other opioids in connection with oxidative stress. Generally speaking, morphine is thought to contribute to oxidative stress either by promoting the formation of free radicals or by reducing the activity of different antioxidant components in target cells. Together, these effects can play an important role in the development of oxidative stress. Akpınar et al. found that dexmedetomidine shows an anti-oxidant effect by regulating the transient receptor potential melastatin-2 (TRPM2) and Transient receptor potential vanilloid 1 (TRPV1) channel activations that cause oxidative stress in neuronal injury.35
Previous studies have shown that dexmedetomidine reduces ischaemia/reperfusion injury in rats by reducing oxidative stress and cell apoptosis, and exhibits a neuroprotective effect in intracerebral haemorrhage.36,37 In our literature review, however, we failed to find any studies on the effects of dexmedetomidine on oxidative stress and apoptosis arising from morphine tolerance. We concluded that dexmedetomidine administered alone showed antioxidant properties and attenuated the increase in oxidative stress caused by morphine and morphine tolerance. Akpınar et al. showed that different doses of dexmedetomidine administered to rats with cerebral ischaemia reduced cerebral oxidative stress in and inhibited expression of Caspase-3 and Caspase-9.36 Li et al. reported that dexmedetomidine demonstrated an anti-inflammatory effect by inhibiting the NLRP3/Caspase-1 cascade, which was activated by stimulation of lipopolysaccharide (LPS) and adenine-triphosphate (ATP).38
Our study has shown that morphine tolerance induced apoptosis in DRG by increasing Caspase-3 and Caspase-9. Although single-dose morphine increased inflammation and overall oxidant status and decreased overall anti-oxidant status in DRG, it did not induce apoptosis. This could be due to the existence of an apoptosis threshold in DRG after morphine administration. Dexmedetomidine reduced apoptosis tolerance by reducing Caspase-3 and Caspase-9 in DRG. The anti-inflammatory and anti-oxidant effect of dexmedetomidine may explain the anti-apoptotic effect of dexmedetomidine for lowering morphine tolerance.
Our study has some limitations. We believe that sc injection of 10 mg/kg morphine twice daily for 5 days is sufficient to develop morphine tolerance. However, we did not confirm this by measuring the level of analgesia after each injection. Inflammatory parameters, TNF and IL-1, were only measured using ELISA kits, but additional tests, such as western blot, would have further confirmed the accuracy of our measurements.
Tolerance of the analgesic effect of morphine is common in clinical practice, especially in patients with chronic pain. To overcome this, higher doses of morphine are administered, and this can lead to serious adverse effects such as respiratory depression. To reduce tolerance to morphine in patients with chronic pain, a solution containing morphine plus a tolerance-modulating adjunct should be developed in the future. The results our study show that dexmedetomidine could be just such an adjunct, but human studies are be needed to confirm this hypothesis. However, iv dexmedetomidine can have serious side effects, such as hypotension, bradycardia, and respiratory depression, so the use of dexmedetomidine to reduce morphine tolerance should probably be restricted to intensive care units with close hemodynamic monitoring.
ConclusionIn conclusion, dexmedetomidine has antinociceptive, antioxidative and anti-apoptotic effects. It increases the analgesic effect of morphine while preventing morphine tolerance by reducing TNF and IL-1 levels. For these reasons, dexmedetomidine could be used to mitigate the adverse events associated with morphine tolerance.
Informed consentThe Sivas Cumhuriyet University Animal Ethics Committee approved the experimental protocols for this study (Approval No: 65202830-050.04.04-435).
Data sharing statementThe clinical trial data of this article are available upon reasonable request to the corresponding author.
Conflict of interestNo potential conflict of interest was reported by the authors.
The authors would like to thank the management of Sivas Cumhuriyet University, Sivas, Turkey for providing the necessary facilities to conduct this study.