Transient osteoporosis of the hip (THO) is a rare disease of unknown pathogenesis that has traditionally been considered an early and reversible stage of avascular necrosis (AN). Thrombophilia or familial hypofibrinolysis is considered a risk factor for the development of AN and THO. Factor V Leiden is one of the most common hereditary hypercoagulability disorders.
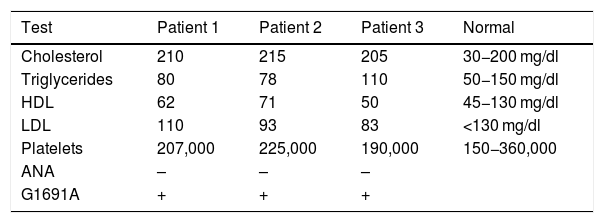
Clinical casesCase series study. The development and course of 3 THO cases in 3 siblings (two males and one female) aged between 40 and 43 years are described consecutively. Clinical and nuclear magnetic resonance imaging (MRI) studies confirmed the diagnosis of THO and ruled out the presence of AN. The G1691A mutation of factor v Leiden was positive in all cases. The clinical and radiological outcome was favourable, with healing without sequelae and disappearance of bone oedema on control MRI at 6 months in all cases.
DiscussionThe results of this study support the ischaemic aetiology and establish HTO as an early and reversable stage of hip AN. Factor V Leiden causes a state of hypercoagulability and hypofibrinolysis that encourages the development of THO due to ischaemic causes.
ConclusionsThis study outlines the first familiar description of factor v Leiden-linked THO.
La osteoporosis transitoria de cadera (OTC) es una enfermedad poco frecuente y de patogenia desconocida que ha sido considerada tradicionalmente una fase precoz y reversible de la necrosis avascular (NA). La trombofilia o la hipofibrinólisis familiar se considera un factor de riesgo para el desarrollo de NA y OTC. Dentro de los trastornos heredados de hipercoagulabilidad, el factor v de Leiden es uno de los más prevalentes.
CasosclínicosEstudio tipo serie de casos. Se describen el desarrollo y la evolución de 3 casos de OTC en 3 hermanos (2 varones y una mujer) con edades comprendidas entre los 40 y 43 años de forma consecutiva. El estudio clínico y de imagen con resonancia magnética nuclear (RM) confirmó el diagnóstico de OTC y descartó la presencia de NA. La mutación G1691A para el factor v de Leiden fue positiva en todos los casos. La evolución clínica y radiológica fue favorable, con curación sin secuelas y desaparición del edema óseo en la RM de control a los 6 meses en todos los casos.
DiscusiónLos resultados del presente estudio apoyan la etiología isquémica y establecen a la OTC como una fase precoz y reversible de la NA de cadera. El factor v de Leiden promueve un estado de hipercoagulabilidad e hipofibrinólisis que favorece el desarrollo de la OTC por causa isquémica.
ConclusionesEl presente estudio desarrolla la primera descripción familiar de la OTC ligada al factor v de Leiden.
In 1959, Curtiss and Kincaid first described 3 cases of hip or thigh pain in the third trimester of pregnancy that presented radiologically as a subtle demineralisation of the femoral head and, to a lesser extent, of the femoral neck and acetabulum, and which recovered spontaneously a few months later.1 In 1988, Wilson et al. used the term “transient bone marrow oedema” to refer to patients with hip and knee pain who presented with osteopenia or normal bone mineral density on densitometry.2
The term “bone marrow oedema syndrome” (BMOS) encompasses 2 clinical entities, transient osteoporosis of the hip (TOH) and regional migratory osteoporosis (RMO). TOH is more common in middle-aged men. In women, it is most often found in the third trimester of pregnancy. It usually affects the proximal area of the femur and rarely the acetabulum, and the gold standard for diagnosis is magnetic resonance imaging (MRI). Although a matter of debate, TOH can be considered an early stage of avascular necrosis (AN). However, while TOH generally resolves without sequelae, AN is normally an irreversible and progressive disease, which results in interrupted vascular supply to the femoral head, occasionally causing permanent loss of joint function.
The anticoagulant effect of protein C was first described by Mammen et al.3 Inherited resistance to activated protein C was described by Dahlbäck et al. and associated with familial thrombophilia.4 More frequently, resistance to activated protein C is caused by a genetic mutation that results in loss of the binding site to factor V, giving rise to what has been termed factor V Leiden, a severe hypercoagulability disorder. Factor V Leiden has been identified as a risk factor for the development of AN.5–8 This article provides a first description of factor V Leiden mutation in three siblings with TOH.
Clinical casesA case-series study. Three siblings, 2 males and one female aged between 40 and 42 years, were attended consecutively in consultation for disabling hip pain of approximately one month’s duration. None of them had risk factors for developing AN (habitual alcohol consumption, steroid treatment or hyperlipidaemia) or a history of trauma that could have caused it. None of the siblings had a previous history of deep vein thrombosis, although the female patient had a previous history of repeated miscarriages.
Measurement of lipids, cholesterol, platelets and G1691A mutationLipids, cholesterol levels, platelets and antinuclear antibodies (ANA) were quantified in peripheral blood using standard enzymatic methods (the detailed test parameters are shown in Table 1).
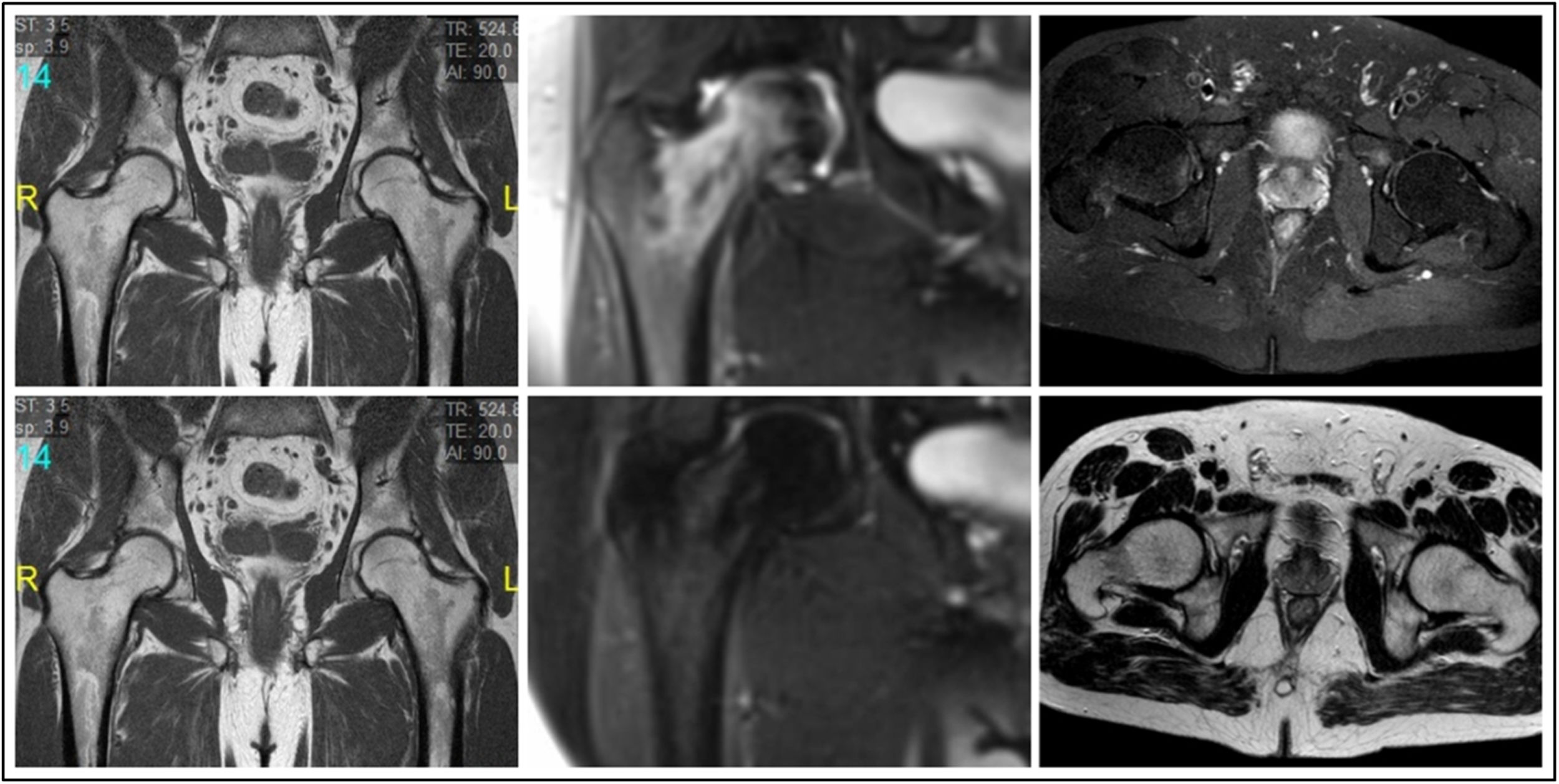
Nuclear magnetic resonanceMRI studies were performed on a 3.0 Tesla system (Magnetom Expert, Siemens, Erlangen, Germany). In all 3 cases, MRI revealed the presence of bone marrow oedema (BMO) in the right femoral head and intertrochanteric area, without signs of associated bone necrosis (Fig. 1).
Genetic studyThe female patient’s history of repeated miscarriage motivated the genetic study. Given the identical symptoms in the male patients, and ruling out the presence of known risk factors, a genetic study was recommended for all the cases.
The G1691A mutation was detected by extracting DNA from peripheral blood using the NucleoSpin (Manchery-Nagel) kit for extracting genomic DNA from tissue samples. The G1691A mutation was positive in all three cases.
Treatment and progressionAfter overall assessment of the patients, they were defined as cases of TOH and were treated conservatively by keeping the joint fully non-weight bearing for 4 weeks combined with magnetotherapy, conventional analgesic treatment and gentle physiotherapy to prevent contractures, followed by partial weight bearing with a stick for 4 further weeks. No antiresorptive drugs were used. Control MRI at 6 months showed full resolution of the oedema in all cases with no progression to AN (Fig. 1). Clinically, the patients made a full functional recovery with no sequelae.
DiscussionResistance to activated protein C is the most common genetic defect in patients with thrombosis, particularly thrombosis where there is an association with family members.9 The substitution of arginine at nucleotide position 506 by glutamine results in an alteration in the activated protein C binding site to factor V, which is known as Leiden factor V.
The limitations of this study are the small number of cases described and its descriptive nature. It is, however, the first description of familial TOH linked to factor V Leiden. Factor V Leiden could also behave as a confounder of another aetiological factor that is unknown at this time.
States of hypercoagulability and hypofibrinolysis were established as risk factors for the development of BMOS by Berger et al., who related elevated levels of lipoprotein (a) and activated plasminogen inhibitor with the development of the disease.10 The first description of familial BMOS was by Berger et al.11 Of the 3 patients described, 2 were sisters and the third was the daughter of one of them. Plasma lipoprotein (a) levels were elevated in all 3 patients. A relationship between elevated lipoprotein (a) levels and the development of BMOS and AN due to inadequate lysis of intraosseous thrombi and subsequent increase in venous pressure was proposed. Most cases of familial AN of the femoral head are associated with Gaucher disease, sickle cell anaemia or thrombophilia, and familial hypofibrinolysis, although sporadic cases with no known predisposing factors have been published. Glueck et al. conducted a study to analyse whether inherited thrombophilia and hypofibrinolysis were risk factors for the development of femoral head AN in patients with idiopathic or high-dose steroid-associated AN.7 The presence of thrombophilia and hereditary hypofibrinolysis was more common in patients with idiopathic or secondary AN than in healthy subjects, and therefore they were considered risk factors for the development of the disease.
Factor V Leiden has been identified as a risk factor for the development of AN in numerous studies.5–8 Björkman et al. concluded that factor V and prothrombin gene 20210A mutations, as well as thromboembolic events were significantly more common in patients with idiopathic NA, NA secondary to steroid use or alcohol-induced AN than in the healthy population.5 Glueck et al. highlighted factor V Leiden as a risk factor for the development of AN, being present in 9.3% of patients with idiopathic NA and 9.6% of patients with secondary AN.6 In another similar study, Glueck et al. found that factor V Leiden mutation was more frequent in patients with secondary multifocal AN than in healthy controls.8 In these familial hypercoagulability states it has also been shown that testosterone therapy can worsen the progression of AN.12
ConclusionsWe present the first description of transient familial factor V Leiden-linked TOH. The results of this study support an ischaemic aetiology and establish TOH as an early and reversible stage of AN of the hip. Factor V Leiden promotes a state of hypercoagulablity and hypofibrinolysis that favours the development of TOH of ischaemic cause.
Level of evidenceLevel of evidence IV.
Conflict of interestsThe authors have no conflict of interest to declare.
Please cite this article as: Serrano-Toledano D, del Río-Arteaga M, Ribera-Zabalbeascoa J. Osteoporosis transitoria de cadera familiar ligada al factor v de Leiden. Rev Esp Cir Ortop Traumatol. 2020;64:286–289.