Case report
A 73-year-old Spanish male with prosthetic valve dysfunction showing paravalvular leak and severe insufficiency presented to the Hospital 12 de Octubre on October 14, 2003, for a replacement of his prosthetic mitral valve by a mechanic prosthesis St. Jude no. 27. The patient had a history of severe COPD, treated with bronchodilators and corticosteroids, and mitral stenosis of long duration. In 1979, he underwent a replacement of his mitral valve with a mechanical prosthetic valve. In 1996, after a perivalvular leak, he underwent a second replacement with a new Carbomedics no. 27 prosthetic valve. Upon hospital admission, the patient showed silent ischemic cardiopathy and moderate pulmonary hypertension. His left ventricular ejection fraction was normal (> 59). An echocardiogram revealed the presence of a mass compatible with a thrombus of 16 mm × 7 mm in the auricular region. Within the first 72 h after the operation he developed acute renal insufficiency of multiple origins with oliguria (< 0.5 ml/kg h) and creatinine elevation (maximum 2.35 mg/100 ml). At the same time he presented with respiratory insufficiency with acute pulmonary oedema, and a bilateral neumothorax, probably related to a bronchopleural fistule and massive left atelectasis. A tracheothomy was performed on October 29. Due to a suspicion of preoperatory endocarditis caused by a thrombus on the prosthetic valve, the patient was treated with vancomycin and gentamycin. After the operation, gentamycin was suspended due to its nephrotoxicity and vancomycin was substituted by linezolid. In the histological examination of the withdrawn mitral prosthesis thrombus, a filamentous septate fungus was observed (Fig. 1). Repeat cultures of that material on Sabouraud glucose agar yielded the growth of an Acremonium sp. Antibiotic treatment was suspended and caspofungin was administered i.v. during 5 days (70 mg the two first days and 50 mg subsequently), which was changed to voriconazole (6 mg/kg day the first day followed by 4 mg/kg day) until November 11. Blood cultures were negative. On October 29, nosocomial pneumonia was suspected based on fever, leucocytosis, hemodynamic and respiratory impairment, purulent secretions, and infiltrates seen by radiograph. New empirical treatment with linezolid and ceftazidime was initiated. Over the following days, Staphylococcus aureus and Stenotrophomonas maltophilia were isolated from respiratory secretions and the patient was treated with linezolid and clotrimoxazole. On November 23, Klebsiella pneumoniae was isolated from urine and blood cultures, and the tip of a catheter, and treatment with aztreonam was initiated. Concurrently, treatment with sodium heparin, initiated after the operation, had to be suspended on several occasions due to digestive haemorrhages. As a consequence of all these complications, the patient developed multiorganic dysfunction with polineuropathy and renal impairment, which required assisted venous drainage and furosemida at low doses. On December 17, the patient suffered septic shock and his clinical status worsened progressively with renal, hemodynamic and respiratory impairment. Microbiological cultures were all negative with the exception of bronchial secretion cultures, where Pseudomonas sp. was cultured. Believing it to be nosocomial pneumonia, treatment with amoxycillin/clavulanic acid 2 g/8 h was started, which was changed to with levofloxacin. Thoracicradiographs did not reveal the presence of any infiltrate. On December 22, the patient died as a result of respiratory insufficiency and an asystole. Autopsy was denied by his relatives.
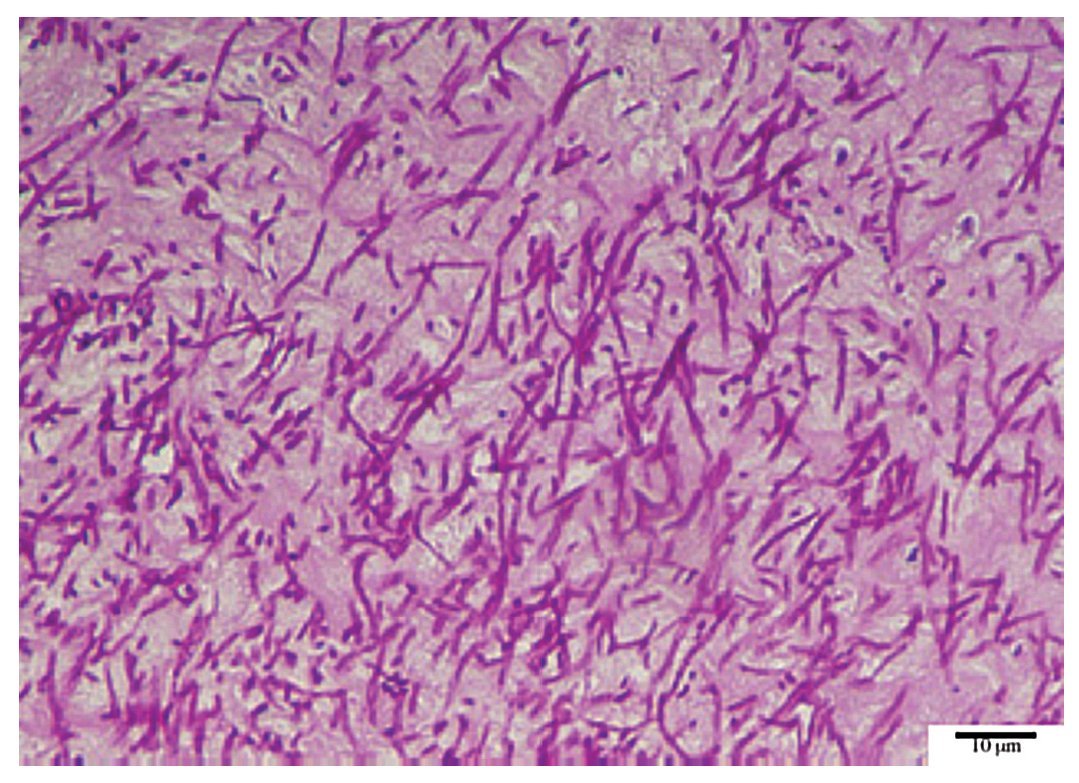
Figure 1. Thrombus from the prosthetic mitral valve stained with periodic acid- Schiff staining showing branched and septate hyphae.
Repeat cultures of the thrombus from the prosthetic mitral valve on Sabouraud dextrose agar grew numerous colonies of a filamentous fungus morphologically identified as Acremonium strictum W. Gams2,16. The fungus was deposited in the Medicine School of the Rovira i Virgili University, Tarragona (Spain), as FMR 8303. This species is characterized by moist to slimy, orange to salmon colonies, with conidiophores usually reduced to simple phialides arising from the submerged or slightly fasciculate aerial mycelium. The phialides are acicular, 20-65× 1.4-2.5 µm, and produce hyaline, cylindrical to ellipsoidal, 3.3-5.5× 1-2 µm conidia grouped in slimy heads (Fig. 2). To confirm this identification, a fragment of about 531 bp corresponding to the ITS region of the rRNA genes of this isolate was sequenced (GenBank accession no. AM990178) and compared with sequences of the GenBank. The search revealed a 100% homology with the strain UW 836 of A. strictum (AY138844).
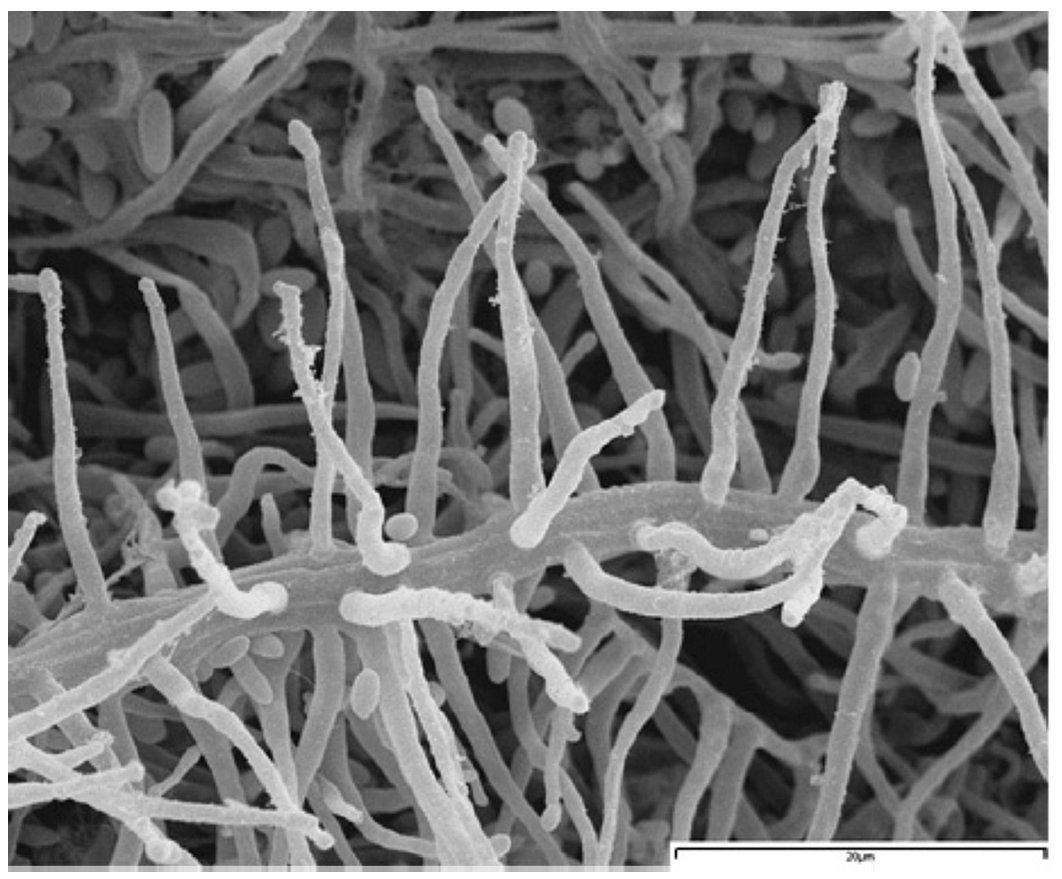
Figure 2. A hyphal strand with numerous conidiogenous cells of Acremonium strictum (FMR 8303) under scanning electron microscopy.
Susceptibility testing of the clinical isolate was performed in duplicate using a microdilution test and following the Clinical and Laboratory Standards Institute (CLSI) guidelines11. The MICs for the antifungals tested (in µg/ml) were as follows: amphotericin B 2, itraconazole > 16, posaconazole 2, terbinafine 0.25, micafungin > 16, voriconazole 2 and ravuconazole 16.
This report documents only the presence of A. strictum colonizing the prosthetic mitral valve. However, considering the virulence of this fungus, it may have had a role in the progressive decline of the patient. It is noteworthy that the sequence of our clinical isolate is identical to that of the strain UW 836, which recently caused a fatal infection in a haematopoietic cell transplant patient in the USA12.
Those authors found a remarkable genetic diversity among clinical isolates of this species when the ITS region and 28S rRNA sequences were analyzed. This seems to indicate that A. strictum may be a complex of phylogenetic species as is seen in many other pathogenic fungi, such as Pseudallescheria boydii6 or Sporothrix schenckii9, previously considered homogeneous species.
A review of the clinical cases of infections by Acremonium found eight cases attributed to A. strictum, reported up to 2002, and in five of these, the patient died7,8. Considering the difficulties in the identification of this fungus and the fact that in numerous cases attributed to Acremonium are not identified to the species level, it is likely that disease by A. strictium is underreported. In recent years, several new cases attributed to this fungus have been reported, i.e. localized infections affecting immunocompetent patients1,3,13,15, invasive infections in immunosuppressed patients5,10 and even some affecting animals14.
The antifungal susceptibility for this case isolate is similar to that seen in the case of Novicki et al12. Both isolates showed an itraconazole MIC of > 8 µg/ml and an amphotericin B MIC of 2 µg/ml. In contrast, response to other azoles was considerably poorer than that indicated by other authors. Miyakis et al10, reported MICs for voriconazole and posaconazole of 0.25 and 0.03 µg/ml, respectively. These significant differences in MIC data among the isolates of A. strictum tested may be explained by the existence of an A. strictum species complex, with individual strains displaying different antifungal susceptibilities. In general, however, limited case reports correlating in vitro data with clinical efficacy suggest an organism refractory to antifungal therapy. This resistance is similar to that seen in Fusarium spp., with which Acremonium shares numerous morphological and pathological characteristics. However, it is encouraging to note that on a few occasions, A. strictum has responded to voriconazole in an invasive infection in a bone marrow transplant recipient10 and to posaconazole in a pulmonary infection in a leukemic patient8. Additional studies involving more clinical strains are needed to confirm these promising data. Although in vitro data suggest that new azoles may be effective against some Acremonium isolates4, it is unknown against which species, as A. strictum most likely represents a species complex of diverse and possibly poorly related species.
This case highlights the fact that a fungus such as A. strictum, which is highly resistant to antifungals and has proven ability to invade the debilitated host, can colonize a mechanical prosthetic valve. Such colonization may also constitute an important risk factor for the development of an invasive infection. This fungus should be added to the list of species able to produce fungal endocarditis.
* Corresponding author.
E-mail address: josep.cano@urv.cat (J. Cano).
ARTICLE INFO
Article history:
Received May 5, 2008
Accepted July 23, 2008