Available evidence suggests that cimetidine is a reproductive toxicant that induces sexual and testicular dysfunction. Ocimum gratissimum (OG) is globally consumed for medicinal and nutritional purposes. To determine the modulating role of aqueous leaf extract of Ocimum gratissimum on cimetidine-induced gonado-toxicity, sexually mature male rats were randomized into four groups of six (n=6) rats each. Group A: control given 2ml distilled water. Group B received 500mg/kg body weight (bwt) of OG extract, Group C received 50mg/kg bwt cimetidine, and group D received 50mg/kg bwt of cimetidine+500mg/kg bwt OG extract once daily for 8 weeks via gastric gavage. Parameters tested include sperm parameters, testosterone (TT), luteinizing hormone (LH), follicle stimulating hormone (FSH) and prolactin, testicular alkaline phosphatase (ALP), acid phosphatase (ACP), lactate dehydrogenase (LDH), protein, cholesterol, glycogen, sexual behavioural parameters, and testicular histology.
ResultsThere were depletions in the seminiferous epithelium, decreased sperm quality, TT, LH, and FSH, testicular enzymes, protein, cholesterol, glycogen, and sexual behaviour increase in animals treated with cimetidine only compared to control. OG restored and improved sexual behaviour and libido as evident from increased frequencies of mount, intromission, ejaculation, and ejaculatory latency. Mount latencies, intromission, post-ejaculation, and prolactin were significantly decreased. The significantly decreased testicular activities of ALP, ACP, LDH and protein, cholesterol, glycogen concentrations, TT, LH and FSH were increased by OG administration.
ConclusionOcimum gratissimum attenuated the deleterious effects of cimetidine on the testis, protected the seminiferous epithelium, restored, and boosted sexual competence, and promoted spermatogenesis.
La evidencia disponible sugiere que la cimetidina es un tóxico reproductivo, que induce la disfunción sexual y testicular. Ocimum gratissimum (OG) se ha consumido a nivel global con fines medicinales y nutricionales. Para determinar el rol modulador del extracto acuoso de las hojas de OG en la toxicidad en gónada inducida por cimetidina distribuyeron aleatoriamente ratas macho maduras en 4 grupos de 6 individuos (n=6). El grupo A o grupo control a quien se administraron 2ml de agua destilada, el grupo B que recibió 500mg/kg de peso corporal (BWT) de extracto de OG, el grupo C que recibió 50mg/kg BWT de cimetidina y el grupo D que recibió 50mg/kg BWT de cimetidina+500mg/kg BWT de extracto de OG una vez al día durante 8 semanas mediante sonda gástrica. Los parámetros probados incluyeron: parámetros de esperma, testosterona (TT), hormona luteinizante (HL), hormona folículo-estimulante (FSH) y prolactina, fosfatasa alcalina testicular (ALP), fosfatasa ácida (ACP), lactato deshidrogenasa (LDH), proteína, colesterol, glucógeno, parámetros del comportamiento sexual e histología testicular.
ResultadosSe observó reducción del epitelio seminífero, disminución de la calidad espermática, TT, HL y FSH, enzimas testiculares, proteína, colesterol, glucógeno e incremento del comportamiento sexual en los animales tratados con cimetidina en comparación con el grupo control. OG restauró y mejoró el comportamiento sexual y la libido, según evidenció el incremento de las frecuencias de monta, intromisión, eyaculación y latencia eyaculatoria. Las latencias de monta, intromisión, posteyaculación y prolactina disminuyeron significativamente. Las actividades testiculares significativamente disminuidas de ALP, ACP, LDH y proteína, colesterol, concentraciones de glucógeno, TT, HL y FSH se incrementaron con la administración de OG.
ConclusiónOcimum gratissimum atenuó los efectos perjudiciales de cimetidina en los testículos, protegió el epitelio seminífero, restauró y potenció la competencia sexual y promovió la espermatogénesis.
Cimetidine belong to a group of medicines called H2-receptor antagonists which act to decrease the natural production of acid in the stomach.1 Cimetidine have been prescribed to treat any of the following: Ulcers in the stomach (gastric ulcer) or in the upper part of the intestine (duodenum ulcer), acid from the stomach escaping into the food pipe causing pain, inflammation and heartburn (oesophageal reflux disease), excess acid in the stomach caused by a tumor in the pancreas (Zollinger-Ellison syndrome). Ulcers in the stomach or duodenum which may be caused by non-steroidal anti-inflammatory drugs, often used to reduce pain, fever and inflammation.2 It acts by competing for H2 receptors in the gastric parietal cells.3 It has antitumor and anticancer properties because of its antiangiogenesis and anti-adhesion effects and its ability to inhibit tumor cell propagation and metastasis.4 It also has a hepatoprotective effect against hepatotoxins as isoniazide and rifampicin.5 The main adverse effect of cimetidine is its antiandrogenic.6 Cimetidine has been reported to cause some adverse effects in male, such as loss of libido, impotence, and gynecomastia7 which has been related to an antiandrogenic effect.8 Reduction of testis, prostate, and seminal vesicles weight and changes in the hormonal levels have been observed,9 confirming the antiandrogenic effect of cimetidine drug.10 In testes, this drug has caused significant histopathological disorders in the seminiferous tubules, including reduction of the tubular diameter and epithelial area due to detachment11 and loss of germ cells by apoptosis.12 This tubular alteration seems to be a consequence of the interference of cimetidine on the peritubular tissue and Sertoli cell death.13 Recently, studies have demonstrated that cimetidine causes testicular microvasculature atrophy,14 reduction in the number of Leydig cells due to apoptosis, and decrease in the testosterone serum levels.15 These vascular and hormonal changes seem to be the main causative effects of the seminiferous tubules’ structural disruption.16 The testis is the major male reproductive organ, which is primarily responsible for sperm production (spermatogenesis) and androgen synthesis (steroidogenesis). Spermatogenesis takes place primarily in the seminiferous tubules while steroidogenesis occurs in the Leydig cells of the testis and both processes are necessary for fertility in the male. Cimetidine has been demonstrated by Gill et al.,17 to be a testicular toxicant and a number of studies have reported on its high potential of causing reproductive dysfunction in male.
For decade, man has depended on ingesting herbs as supplements for body preservation as well as therapy for several ailments such as diarrhoea, lower respiratory tract infection, common cold and skin infections.18 In recent years the ingestion of these natural products has received incredible global endorsement.19 Factors attributed to this include the desire to naturally keep the body healthy.20
Ocimum gratissimum, commonly referred to as ‘scent leaves’, and also known as Ramtulsi in Hindi, Bengali, and Gujrati; Fever leaf in English; Banjere in Punjabi; Rama tulsi in Malyalam; Elumicham tulasi in Tamil; Nimma tulsi in Kannad,21 it is a perennial herb that belongs to the family Lamiaceae.22 It is originated from Asia and Africa.23 It is used as a traditional vegetable condiment and oral care products in Nigeria and other parts of the world,24 it had been shown to possess numerous pharmacological properties hence its use in traditional or alternative medicine.25 These properties include antioxidant, anti-anemic, antidiarrhoeal and protective effects on hepato-renal indices.26Ocimum gratissimum topically applied either in dry or fresh form was found to be very active against dermatophytes of both scalp and hands after two weeks of twice daily application while oral treatment showed no improvement but did expel worms therefore it has clinical application in the treatment of superficial fungi infection.27Ocimum gratissimum is used by the Igbo community of south eastern Nigeria in management of the baby's cord, to keep the wound surfaces sterile.28 In Southern part of Nigeria, crude aqueous extract of Ocimum gratissimum is commonly employed in the treatment of diarrhea.29 Essential oils obtained from Ocimum gratissimum species showed various medicinal potentials in chemopreventive, anticarcinogenic, free radical scavenging, and radioprotective uses.30 Also, ethanolic extract of Ocimum gratissimum leaf revealed significant chemopreventive effects on chemical-induced papilloma genesis by modulating metabolizing enzymes such as cytochrome P450, glutathione-s-transferase, and aryl hydrocarbon hydroxylase.31 The plant leaf extract is used in alternative medicine for the management of erectile dysfunction.32 However, there is limited information on the possible mechanisms of actions of bioactive component of Ocimum gratissimum on the cimetidine induced gonado-toxicity. This study therefore investigated the modulating role of aqueous leaf extract of Ocimum gratissimum on cimetidine-induced gonado-toxicity in rat.
MethodsChemicals, drugs, reagents and assay kitsCimetidine was purchases from FEGTOCHI Pharmacy, Akure, Nigeria. While estradiol benzoate and progesterone were procured from Sigma Chemical (St. Louis, USA) and Shalina Laboratories (Mumbai, India), respectively. Assay kits for protein, cholesterol, glycogen, gamma glutamyl transferase (GGT), alkaline phosphatase (ALP), acid phosphatase (ACP), and lactate dehydrogenase (LDH) were products of Randox Laboratories Limited (Co Antrim, United Kingdom) while those of testosterone, follicle-stimulating hormone (FSH) and luteinizing hormone (LH) were obtained from Monobind Inc. (California, USA). All other reagents used were of analytical grades.
Plant collection, identification and extract preparationFresh leaves of Ocimum gratissimum collected from Research Farm Federal University of Technology, Akure, Nigeria and were identified authenticated by Omomoh Bernand in herbarium section of Centre for Research and Development (CERAD) of the Federal University of Technology, Akure, Nigeria. FUTA0188 voucher deposited for reference purpose. The leaves were thoroughly washed and oven dried at 37°C for 48h and pulverized into smooth powder. The pulverized sample (850g) was suspended in 1000ml of distilled water with regular agitation for 72h. The solution obtained was filtered and the resulting filtrate was concentrated over water bath (40°C) and yielded 463.21g crude extract corresponding to 58.12% of the residue. The dried crude extract Ocimum gratissimum was kept air-tight and refrigerated before use.
Phytochemical screeningQualitative phytochemical analysis of the aqueous leaf extract of Ocimum gratissimum was done in accordance with Soni and Sosa.33
Experimental animalsSexually mature healthy Sprague Darley rats collected from the breeding stoke Federal University of Technology, Akure, Nigeria and kept in clean metabolic cages placed in a well-ventilated room with optimum condition (temperature 25±2°C, photoperiod; 12h natural light and 12h dark; humidity; 45–50%). They were acclimatized to the animal room condition for 2 weeks during which they had free access to feed and water ad libitum. The cages were cleaned daily and overall treatments were in accordance with the guidelines of National Institute of Health on the care and use of laboratory animals.34
Experimental designSexually mature male rats were randomized into four groups of six (n=6) rats each. Animals in group A served as control were given sterile placebo (2ml distilled water). Group B received 500mg/kg body weight of Ocimum gratissimum extract, Animals in groups C received 50mg/kg body cimetidine and group D treated with 50mg/kg body weight of cimetidine simultaneously with 500mg/kg body weight of Ocimum gratissimum extract. All administrations were done once daily for 8 weeks (Duration of spermatogenesis in rat being 51.6–56 days),3 via gastric gavage.
Copulatory behavior testThe copulatory behavior of male rats was monitored by two trained observers blinded to the experimental design, in a sound-attenuated room. Six male rats from each group were subjected to the sexual behavior test. The test was performed 24h after the last treatment and during the dark phase of the light/dark cycle. A single male rat was placed in a rectangular Plexiglas observation chamber (45cm×40cm×30cm) and allowed to acclimate for 5min. A sexually receptive female rat was then introduced into the chamber. The following parameters of sexual behavior were measured as mount frequency (MF), intromission frequency (IF), ejaculation frequency (EF), mount latency (ML), intromission latency (IL), ejaculation latency (EL) and post-ejaculation latency (PEI) were monitored for 30min post pairing period.35
Induction of oestrus phase in female ratsThe procedures of Tajuddin et al.,36 and OECD37 were adopted in this study. Briefly, female rats were brought to oestrus by consecutive subcutaneous administration of estradiol benzoate (10μg/100g) and progesterone (0.5mg/100g) at 48h and 6h respectively prior to pairing. Confirmation of oestrus phase was done by vaginal smears examination.
Animal sacrifice and sample collectionAt the end of the treatment, rats were weighed, blood samples were collected through orbital venous sinus of live animals with microhematocrit tubes within the hours of 7:00am and 8:00am and immediately centrifuged at 3000×g for 10min for serum separation to estimate reproductive hormone. Thereafter, the rats were euthanized by thiopental (100mg/kg, i.p.). The abdomen was opened through a midline incision and a bilateral orchiectomy was rapidly performed, testes were removed and the testicular weights of each animal were evaluated with an electronic analytical and precision balance CAMRY (EK5055, Indian). The testes volumes were measured by water displacement method. The two testes of each rat were measured, and the average value obtained for each of the two parameters was regarded as one observation. One of the testes of each animal was fixed in Bouin's fluid for histological and morphometric analysis. Serum and the remaining testes of each animal were stored at −25°C for subsequent biochemical assays.
Testicular homogenate preparationAfter 6h of sexual competence study, the rats were euthanized by thiopental (100mg/kg, i.p.). The testes were immediately but diligently isolated from the rats, cleaned and homogenized in ice-cold 0.25M sucrose solution (1:5 w/v). The homogenates were centrifuged at 10,000×g for 10min at 4°C to obtain post-mitochondrial fractions and the resulting supernatant was stored at −20°C to ensure maximum liberation of the testicular fractions.
Measurement of sperm parametersThe rats were anaesthetized with sodium thiopental. A scrotal incision was made to exteriorize the testis and epididymides.The caudal epididymis was carefully removed, blotted free of blood and then placed in a prewarmed Petri dish containing 2.0ml of medium Ham's F-10 medium (Sigma, USA) containing 0.5% bovine serum albumin. After 5min of incubation at 37°C (with 5% CO2). Several incisions were made on it to allow sperm swim out. Semen analysis was carried out immediately using the new improved Neubauer's haemocytometer counting chamber for determination of the concentration of spermatozoa. Sperm motility was also assessed immediately by counting both motile and immotile spermatozoa per unit area at the magnification of 40× according to the World Health Organization38 method. Sperm viability was assessed using eosin-nigrosin test. The percentages of unstained (live) and stained (dead) spermatozoa were calculated by counting 200 spermatozoa per sample. Morphological appearance of normal and abnormal spermatozoa was determined by examining stained smears under the oil immersion (100x) and their percentages were calculated.
Biochemical and hormonal assayThe testicular activities of LDH, ACP, ALP and cholesterol concentration were determined in accordance with the manufacturers’ specifications in the respective manual. While the method of Lowry et al.,39 was adopted in the estimation of testicular protein concentration, that of glycogen was evaluated following the procedure of Kemp and Van Heijningen.40 The serum levels of testosterone, FSH, LH and prolactin were determined using ELISA kits according to the manufacturer's instructions.
Histopathological studyAfter fixation, the specimens were dehydrated in alcohol, cleared in xylene and embedded in paraffin. Paraffin sections of 5-μm thickness were cut by a rotatory microtome, mounted on clean slides and stained with haematoxylin and eosin for histopathological examination
Morphometric analysisMorphometric analysis was done as describe by Akunna et al.,41 Histological slides prepared from Bouin's fluid fixed testes. Before embedding, the sections were placed perpendicular to their long axes, and chosen as “vertical sections”. For each testis, seven “vertical sections” from the polar and the equatorial regions were sampled.42 Seven “vertical sections” per testis were selected by a systematic sampling method that ensured fair distribution between the polar and equatorial regions of each testis. Diameter, perimeter, length, width, roundness, lumen diameter and germinal epithelia height of seminiferous tubules of the testes were estimated with a digimizer software programme. Unbiased numerical estimation of the following morphometric parameters was determined using a systematic random Scheme43: cross-sectional area of the seminiferous tubules (AC); number of profiles of seminiferous tubules per unit area of testis (NA); and numerical density of the seminiferous tubules (NV) were determined. For each stereological parameter (D, AC, NA and NV), five randomly selected fields from all the seven sections of a single testis was viewed, and estimation on each carried out. The average from a total of seventy readings from five fields in seven sections of the two testes of one rat was obtained and recorded. Estimation of volume density of testicular components and number of seminiferous tubules was done on a computer monitor onto which a graph sheet was superimposed and on which slides were projected from an integrated digital microscope.
Statistical analysisThe results were reported as mean±standard error of mean (S.E.M). Instat-3 computer program (v2.04, GraphPad Software Inc., San Diego, CA, USA) was used to analyze the numbers and to evaluate the significant differences; the comparison of means between each two experimental groups was made using one-way ANOVA with Newman–Keuls Multiple Comparison significant difference test. The differences were considered significant if p<0.05.
ResultsPhytochemical screeningQualitative analysis of Ocimum gratissimum leaves shows the presence of flavonoids, tannins, phlobatannins, terpenoids, cardiac glycoside, saponins, alkaloids and steroids (Table 1).
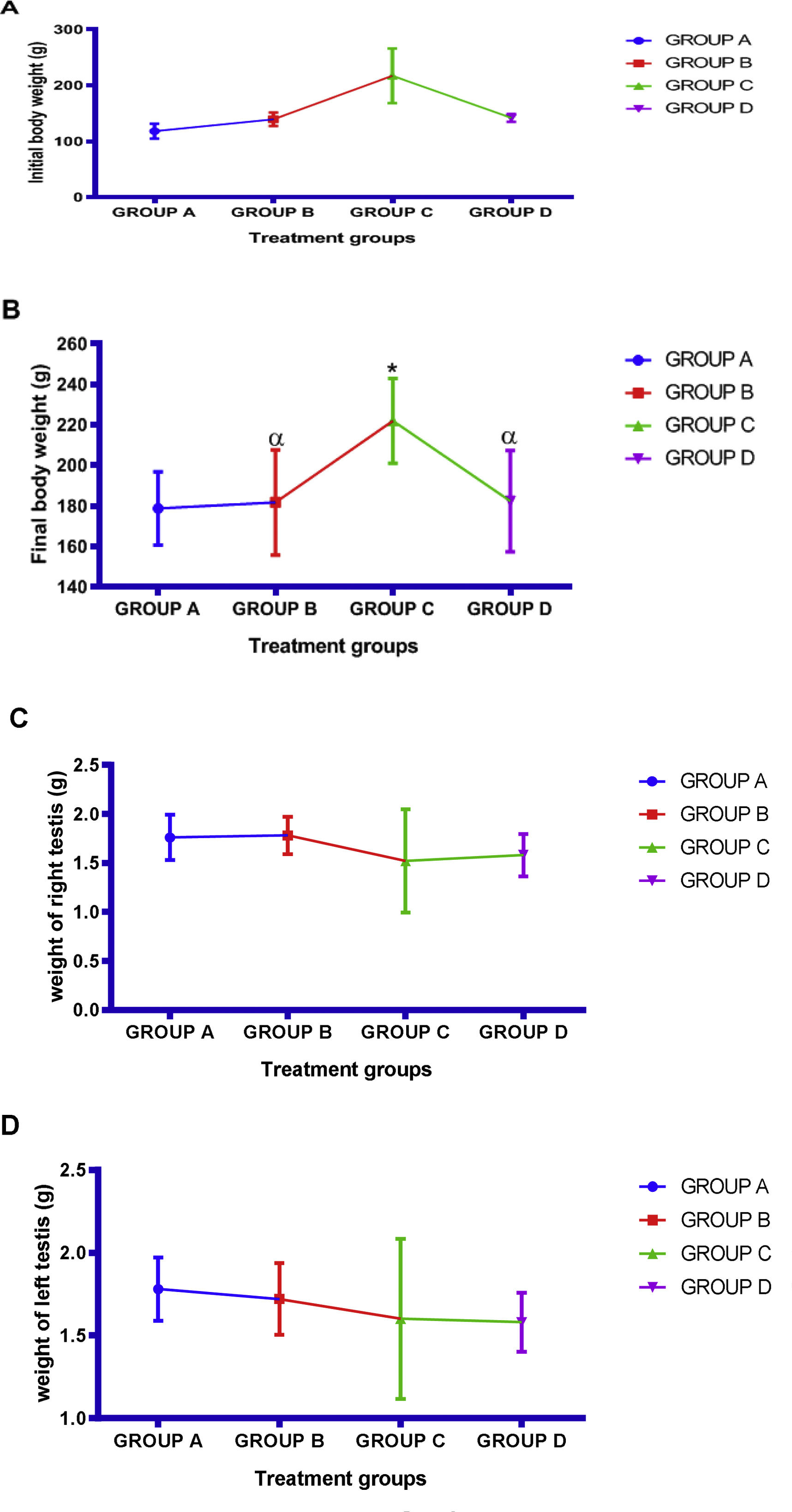
Body weight, testicular weight and physical observationThere was observe significant (p<0.05) increase in final body weight in all the experiment group in comparison with the control group and significant (p<0.05) different decreased in Ocimum gratissimum and cimetidine+Ocimum gratissimum treated group when compared with that of cimetidine treated group. Also, there was significant (p<0.05) increase in mean final body weight in comparison with the initial body weight. There was decrease in mean value of both right and left testes in cimetidine treated group compared with control group and increased in mean value of both right and left testes weight in Ocimum gratissimum and cimetidine+Ocimum gratissimum treated group when compared with that of control group (Fig. 1). During the period of administration, the animals were given intimate observation throughout the period of experiment. During acclimatization, all animals appeared presumably normal with laid hairs and pinkish eyes with good feeding habit. The animals were observed to be using their forelimbs to scratch their mouth on cimetidine administration, the animals got weakened which was observed as a result of reduction of their physical activities.
Effects of aqueous extract of Ocimum gratissimum on body weight (A: Initial body weight, B: Final body weight) and testes weight (C: Right testes weight, D: Left testes weight) in cimetidine induced testicular toxicity in Sprague–Dawley rats, Values are expressed as Mean±S.E.M., n=6 in each group, *: represent significant different from control, α: represent significant different from group C, β: represent significant different from group B at p<0.05, One-Way ANOVA. Group A: 2ml kg/bwt, of distilled water (control), Group B: 500mg/kg bwt of Ocimum gratissimum extract, Group C: 50mg/kg bwt of cimetidine, Group D: 50mg/kg bwt of cimetidine+500mg/kg bwt of Ocimum gratissimum extract.
There was observed decrease in mean value of sperm volume across the group. The mean value of motile sperm count, percentage motility, total count, fast progressivity and normal sperm morphology were significantly (p<0.05) decrease in group D cimetidine treated group as compared with the control group treated with normal saline however significant (p<0.05) increased in mean value of motile sperm count, percentage motility, total count, fast progressivity and normal sperm morphology of group D treated with Ocimum gratissimum and cimetidine when compared with the mean value of group C treated with only cimetidine. Also, there was significant increase in mean value of group B treated with only Ocimum gratissimum in comparison with the corresponding values of the control group. There was significant (p<0.05) increased slow progressivity in cimetidine treated group when compared with the control and significant decreased when compared with cimetidine treated group. An increased in sperm abnormal morphology of cimetidine treated group compared to the corresponding values of the control group. Co administration of cimetidine and Ocimum gratissimum alleviated the changes in abnormal sperm morphology (Table 2, Fig. 2).
Effects of aqueous extract of Ocimum gratissimum on sperm parameters of cimetidine-induced testicular toxicity in Sprague–Dawley rats.
| Groups | Parameters | ||||||
|---|---|---|---|---|---|---|---|
| Progressivity | |||||||
| Volume (ml) | Motile count×106/ml | % motility | Total count×106/ml | Slow | Fast | Live/dead ratioLivability (%) | |
| Group A | 1.74±0.08 | 94.00±2.45, | 163.00±10.20 | 283.60±22.47 | 45.40±1.69 | 54.60±1.69 | 77.40±6.23 |
| Group B | 1.72±0.10 | 138.00±5.83*, α | 232.00±12.41*, α | 395.40±14.63*, α | 45.60±1.63 α | 54.40±1.63α | 91.65±8.12*, α |
| Group C | 1.60±0.22 | 62.00±12.00* | 116.00±13.64* | 181.00±29.93* | 61.40±3.28* | 38.20±3.38* | 40.32±3.54* |
| Group D | 1.58±0.08 | 93.00±3.74α | 186.00±11.66α | 291.00±16.03*, α, β | 44.60±1.60 α | 55.40±1.60 α | 74.43±5.32α |
Values are expressed as Mean±S.E.M., n=6 in each group, *: represent significant different from control, α: represent significant different from group C, β: represent significant different from group B at p<0.05, One-Way ANOVA. Group B: 500mg/kg bwt of Ocimum gratissimum extract, Group C: 50mg/kg bwt of cimetidine, Group D: 50mg/kg bwt of cimetidine+500mg/kg bwt of Ocimum gratissimum extract.
Effects of aqueous extract of Ocimum gratissimum on sperm morphology in cimetidine induced testicular toxicity in Sprague–Dawley rats, Values are expressed as Mean±S.E.M., n=6 in each group, *: represent significant different from control, α: represent significant different from group C, β: represent significant different from group B at p<0.05, One-Way ANOVA. Group A: Control (2ml normal saline), Group B: 500mg/kg bwt of Ocimum gratissimum extract, Group C: 50mg/kg bwt of cimetidine, Group D: 50mg/kg bwt of cimetidine+500mg/kg bwt of Ocimum gratissimum extract. N. morphology: Normal morphology.
There was observed significant (p<0.05) increase in percentage number of live/dead sperm in group B treated Ocimum gratissimum extract in comparison with the value of the control group rats and significant (p<0.05) decreased in percentage mean value of live/dead sperm in group C treated with cimetidine when compare with those of control however group B and group D had significant (p<0.05) higher number of live/dead sperm when compare with that of group C exposed to cimetidine (Table 2).
FSH, LH, testosterone and prolactin levelCompared with the control, animals in the cimetidine treated group had significantly (p<0.05) reduced FSH, LH and testosterone levels. Mean value of TT levels as well as FSH and LH levels in Ocimum gratissimum and cimetidine+Ocimum gratissimum treated group were significantly (p<0.05) increased when compared with that of control group. However, significant (p<0.05) increase in prolactin level was observe in cimetidine exposed group when compare with control group and decrease in mean value of prolactin level in Ocimum gratissimum and cimetidine+Ocimum gratissimum treated group in comparison with those of cimetidine exposed group (Table 3).
Effects of aqueous extract of Ocimum gratissimum on follicle stimulating hormone, luteinizing hormone, testosterone and prolactin level in cimetidine induced testicular toxicity in Sprague–Dawley rats.
| Groups | Parameters | |||
|---|---|---|---|---|
| FSH (miu/ml) | LH (miu/ml) | Testosterone (nmol/L) | Prolactin (nmol/L) | |
| Group A | 19.18±2.56 | 10.28±1.03 | 11.57±1.16 | 4.81±1.47 |
| Group B | 18.71±2.56α | 10.13±0.10α | 11.62±1.17α | 6.02±1.50α |
| Group C | 9.47±1.41* | 5.15±0.80* | 4.30±0.37* | 11.48±1.90* |
| Group D | 22.98±2.53α | 11.44±1.04α | 8.78±0.83α | 5.26±1.06α |
Values are expressed as Mean±S.E.M., n=6 in each group, *: represent significant different from control, α: represent significant different from group C, β: represent significant different from group B at p<0.05, One-Way ANOVA. FSH: Follicle stimulating hormone, LH: Luteinizing Hormone. Miu: Mili international unit, ng: Nanogarmme, Group A: Control (2ml normal saline) Group B: 500mg/kg bwt of Ocimum gratissimum exract, Group C: 50mg/kg bwt of cimetidine, Group D: 50mg/kg bwt of cimetidine+500mg/kg bwt of Ocimum gratissimum extract.
Administration of cimetidine significantly (p<0.05) decrease mean value of MF, IF, EF and EL and increased that of ML, IL and PEL when compared with the mean value of the control group that received normal saline. However, administration of Ocimum gratissimum only and co administration of cimetidine with Ocimum gratissimum significantly (p<0.05) increase mean value of MF, IF, EF and EL and significantly (p<0.05) decrease mean value of ML, IL and PEL in comparison with the cimetidine treated group (Table 4).
Effects of aqueous extract of Ocimum gratissimum on mating behavioural parameters in cimetidine induced testicular toxicity in Sprague–Dawley rats.
| Groups | Mating behavioural parameters | ||||||
|---|---|---|---|---|---|---|---|
| MF | IF | EF | ML | IL | EL | PEL | |
| Group A | 15.04±1.64 | 12.87±1.95 | 4.46±0.83 | 68.49±6.74 | 96.06±21.43 | 132.40±15.68 | 129.90±17.13 |
| Group B | 14.02±1.51α | 11.23±1.84α | 3.75±0.95α | 103.60±9.69α | 115.40±23.09α | 139.80±15.53α | 133.00±16.15α |
| Group C | 7.73±1.33* | 4.35±0.83* | 1.24±0.23* | 139.30±11.61* | 197.50±10.77* | 66.14±10.63* | 221.50±27.91* |
| Group D | 14.03±1.69α | 11.84±1.97α | 4.19±0.83α | 100.00±11.05α | 123.80±23.14α | 146.70±14.94α | 140.20±16.12α |
Values are expressed as Mean±S.E.M., n=6 in each group, *: represent significant different from control, α: represent significant different from group C, β: represent significant different from group B at p<0.05, One-Way ANOVA. MF: Mount frequency, IF: Intromission frequency, EF: Ejaculatory frequency, ML: Mount latency (s), IL: Intromission latency (s), EL: Ejaculatory latency (s), PEL: Post-ejaculatory latency (s), Group A: Control (2ml normal saline) Group B: 500mg/kg bwt of Ocimum gratissimum exract, Group C: 50mg/kg bwt of cimetidine, Group D: 50mg/kg bwt of cimetidine+500mg/kg bwt of Ocimum gratissimum extract.
There was observe significant (p<0.05) decrease in mean value of perimeter, diameter and length of the seminiferous tubules in cimetidine exposed group when compared to the control group. However, Ocimum gratissimum treated group after exposure to cimetidine showed significant changes in their tubular diameter when compared to cimetidine treated group (Table 5). There was a significant decrease (p<0.05) in width and increase (p<0.05) in lumen diameter of the seminiferous tubules in cimetidine treated group (Table 6). Co administration of cimetidine with Ocimum gratissimum significantly increase the mean value of width, and lumen diameter of the seminiferous tubules when compared to the cimetidine exposed group. The mean values of germinal epithelia height, cross-sectional area, number of profiles per unit area and numerical density of seminiferous tubules was decrease in cimetidine treated group. However, there was a significant (p<0.05) increase in germinal height, cross-sectional area, number of profiles per unit area and numerical density in Ocimum gratissimum and cimetidine+Ocimum gratissimum treated group in comparison with to cimetidine only treated group (Table 6).
Effects of aqueous extract of Ocimum gratissimum on length of seminiferous tubule (LST), Diameter (D), perimeter of seminiferous tubules (PST), cross sectional area (AC) and number of profiles per unit area (NA) in cimetidine induced testicular toxicity in Sprague–Dawley rats.
| Groups | Parameters | ||||
|---|---|---|---|---|---|
| LST (μm) | D (μm) | PST (μm) | AC (×103μm3) | NA (×10-8μm−3) | |
| Group A | 351.80±8.39 | 236.00±17.95 | 745.50±79.16 | 20.04±2.43 | 20.62±3.62 |
| Group B | 359.60±8.91α | 220.50±17.60α | 755.10±79.31α | 21.08±2.32α | 21.70±3.59α |
| Group D | 311.50±5.64* | 161.80±19.31* | 399.60±82.79* | 12.20±1.29* | 9.54±1.72* |
| Group E | 358.70±15.59α | 251.10±19.02α | 696.50±84.30α | 28.92±2.69*,α | 24.14±3.54α |
Values are expressed as Mean±S.E.M, n=6 in each group, *: represent significant different from control, α: represent significant different from group C, β: represent significant different from group B at p<0.05, One-Way ANOVA. Group A: Control (2ml normal saline) Group B: 500mg/kg bwt of Ocimum gratissimum exract, Group C: 50mg/kg bwt of cimetidine, Group D: 50mg/kg bwt of cimetidine+500mg/kg bwt of Ocimum gratissimum extract.
Effects of aqueous extract of Ocimum gratissimum on germinal epithelia height (GEH), lumen diameter (LD) of seminiferous tubules, width of seminiferous tubules (WST), roundness of seminiferous tubules (RST), numerical density (NV) in cimetidine induced testicular toxicity in Sprague–Dawley rats.
| Groups | Parameters | ||||
|---|---|---|---|---|---|
| GEH | LD (μm) | WST (μm) | RST (μm) | NV (×10-10μm-2) | |
| Group A | 34.08±2.88 | 74.01±6.33 | 256.90±38.27 | 0.90±0.13 | 8.95±1.78 |
| Group B | 35.22±2.81α | 72.89±6.34α | 260.80±38.64α | 0.89±0.14 | 9.30±1.71 |
| Group D | 11.38±2.51* | 184.80±13.97* | 187.30±34.17* | 0.95±0.13 | 6.12±2.04 |
| Group E | 36.55±2.69α | 140.10±11.05α,β | 297.60±41.72α | 0.96±0.13 | 11.58±1.87 |
Values are expressed as Mean±S.E.M., n=6 in each group, *: represent significant different from control, α: represent significant different from group C, β: represent significant different from group B at p<0.05, One-Way ANOVA. Group A: Control (2ml normal saline) Group B: 500mg/kg bwt of Ocimum gratissimum exract, Group C: 50mg/kg bwt of cimetidine, Group D: 50mg/kg bwt of cimetidine+500mg/kg bwt of Ocimum gratissimum extract.
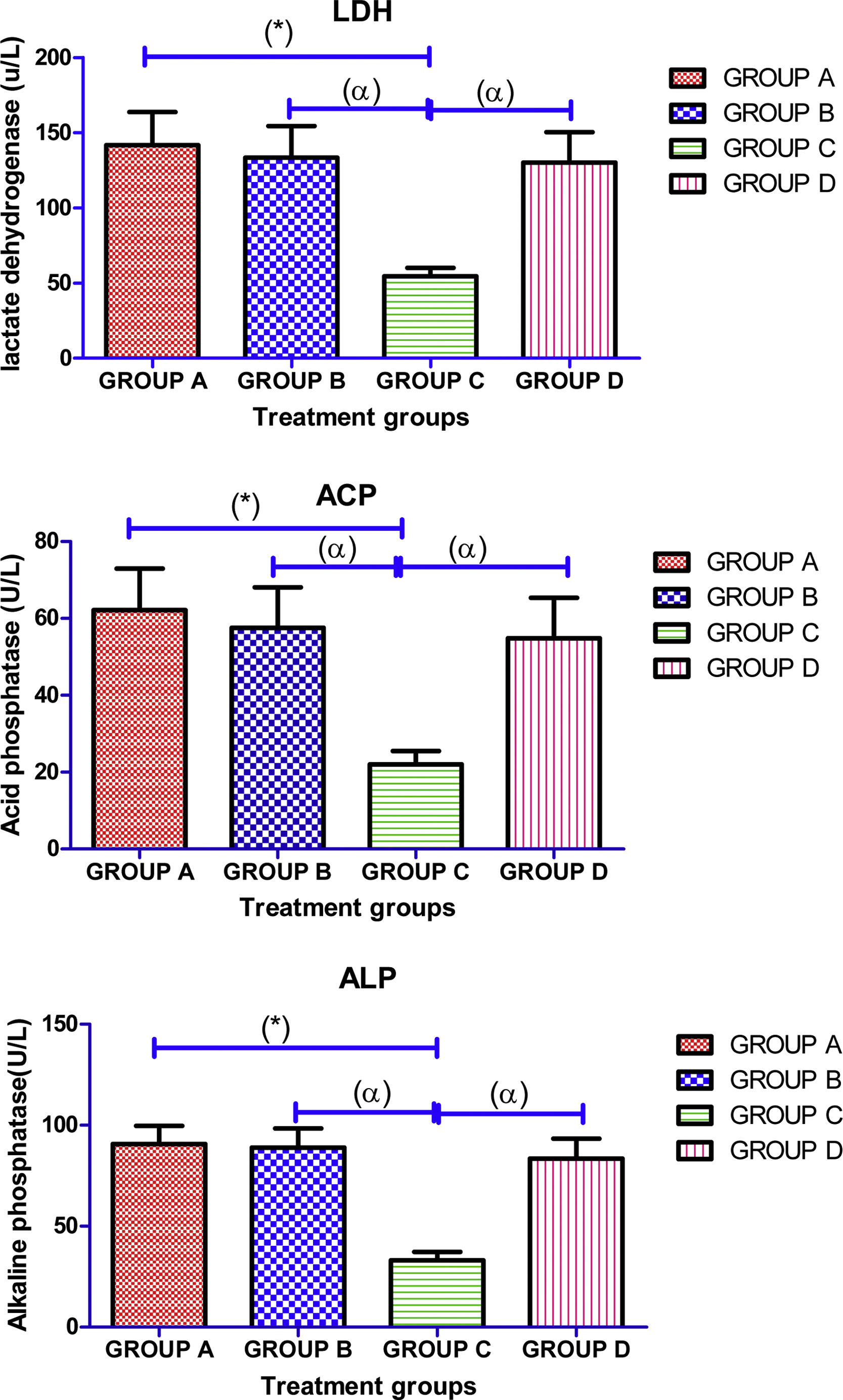
Administration of cimetidine significantly (p<0.05) decreased the mean value of testicular enzymes (LDH, ACP, and ALP) activities in group C when compared with the normal control group. However, there was observe significant (p<0.05) increase in mean value of testicular enzymes (LDH, ACP, and ALP) activities in Ocimum gratissimum and cimetidine+Ocimum gratissimum treated groups in comparison with cimetidine only treated group (Fig. 3).
Effects of aqueous extract of Ocimum gratissimum on testicular activities of some enzymes in cimetidine induced testicular toxicity in Sprague–Dawley rats, Values are expressed as Mean±S.E.M., n=6 in each group, *: represent significant different from control, α: represent significant different from group C, β: represent significant different from group B at p<0.05, One-Way ANOVA. Group A: Control (2ml normal saline), Group B: 500mg/kg bwt of Ocimum gratissimum extract, Group C: 50mg/kg bwt of cimetidine, Group D: 50mg/kg bwt of cimetidine+500mg/kg bwt of Ocimum gratissimum extract. LDH=lactate dehydrogenase, ACP=acid phosphatase, ALP=alkaline phosphatase, bwt.=body weight.
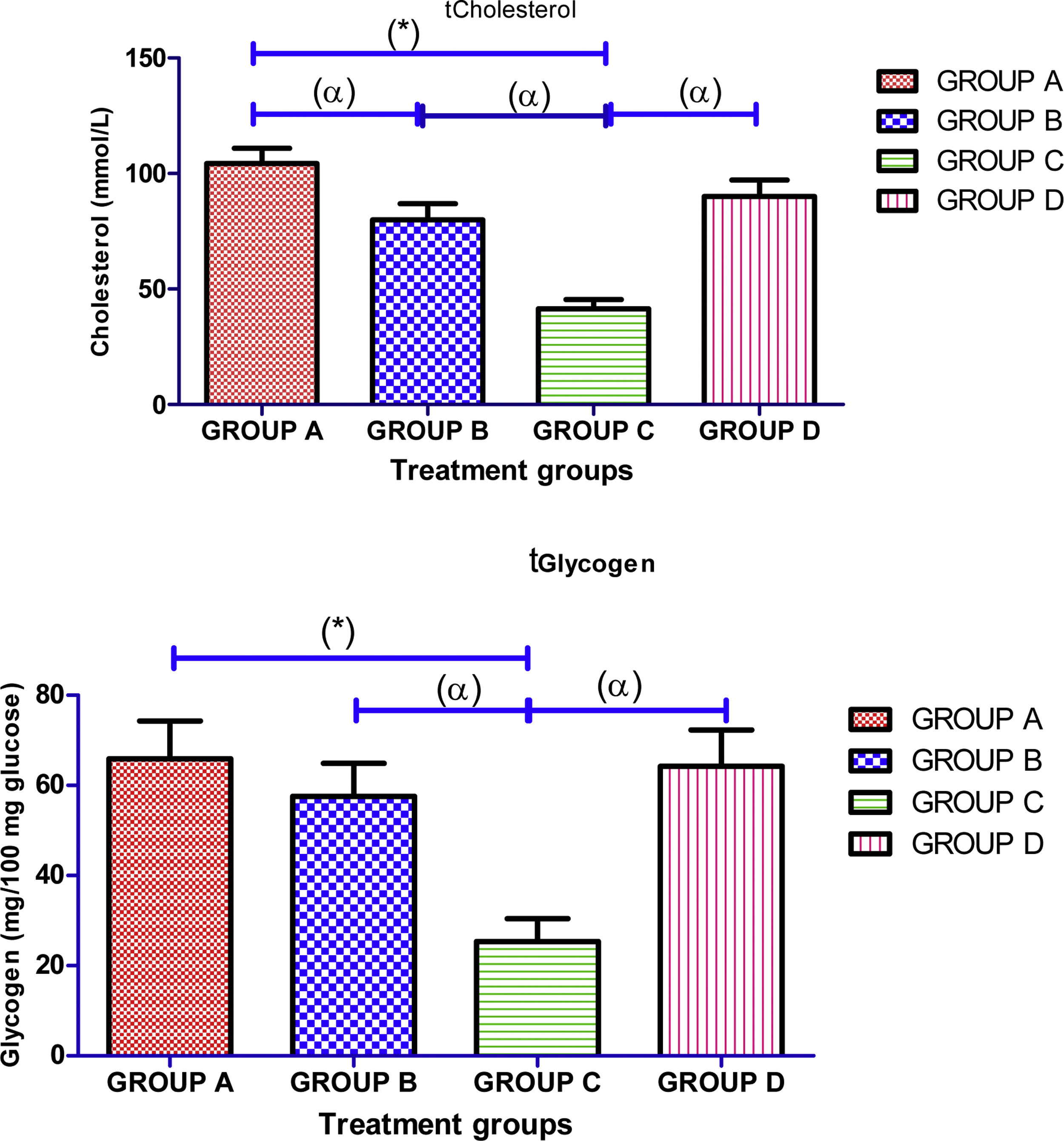
There was significant (p<0.05) decrease in mean value of testicular concentrations of protein, cholesterol and glycogen in cimetidine treated group in comparison with the mean value of control group treated with normal saline. However, administration of Ocimum gratissimum in group B and group D significantly (p<0.05) increased concertation of testicular protein, cholesterol and glycogen when compared with cimetidine treated group (Fig. 4).
Effects of aqueous extract of Ocimum gratissimum on concentrations of testicular cholesterol, protein and glycogen in cimetidine induced testicular toxicity in Sprague–Dawley rats, Values are expressed as Mean±S.E.M., n=6 in each group, *: represent significant different from control, α: represent significant different from group C, β: represent significant different from group B at p<0.05, One-Way ANOVA. Group A: Control (2ml normal saline), Group B: 500mg/kg bwt of Ocimum gratissimum extract, Group C: 50mg/kg bwt of cimetidine, Group D: 50mg/kg bwt of cimetidine+500mg/kg bwt of Ocimum gratissimum extract.
Histopathological examination of testis in group A (control) revealed normal testicular architecture with normal seminiferous tubular and germ cell layers and regular basement membrane. The stratified germinal epithelium is formed of different types of spermatogenic cells; spermatogonia, primary spermatocytes, spermatids, sperms, and Sertoli cells (Fig. 5A). Group B Ocimum gratissimum treated shown normal histo-architecture of testis with normal seminiferous epithelium, normal spermatogenic cell differentiation with numerous spermatozoa in the lumen (Fig. 5B). Group C cimetidine-treated groups, revealed severe degeneration of seminiferous epithelium with relatively wide empty lumen, seminiferous tubular atrophy with epithelial disorganization, reduction in the thickness of the germinal epithelium, vacuolization of spermatogonia, poor differentiation of spermatogenic germ cells, maturation rest and depressed spermatogenesis (Fig. 5C). Group D histological analysis of cimetidine+Ocimum gratissimum treated rats shows normal histology of testis with normal architecture, normal spermatogenesis and numerous spermatozoa in the lumen, some seminiferous tubules have organized lining epithelium with germ cells arranged in concentric layers and sperm tails in its lumen (Fig. 5D).
Histological image of testis stained with (haematoxylin and eosin ×400) method in different study groups. (A) Control group which normal testicular architecture was observed; (B) Treated with Ocimum gratissimum extract in which normal testicular architecture was detected; (C) Treated with cimetidine in which severe testicular injury was noted; (D) Treated with cimetidine+Ocimum gratissimum extract where there was an improvement in the structure of seminiferous tubule. Lumen (L), germinal epithelium (GE), interstitium (I).
Globally Ocimum gratissimum is a well-known traditional medicinal plant and it has the ability to significantly overcoming illness or maintaining health if taken over the time, it acts on the principle of assisting the body's own natural healing process. In this study phytochemical screening of Ocimum gratissimum leaf extract revealed bioactive constituents such as flavonoid, terpenoids, saponin, tannins, steroid and cardiac glycoside which are antioxidant agent against factors causing inflammation, diabetes, cardiac failure, hypertention, bacterial infection, cancer cells, diarrhea, scurvy and membrane lipid perioxidation as shown in Table 1 and reported by Vaidya et al.,44 The phytochemical constituents provide information on the protective functions of Ocimum gratissimum leaf extract against factors that may implicate the testis to cause cell damage.45 The possible protection of the toxicological effects of cimetidine on male reproductive function by Ocimum gratissimum was observed in this study. Cimetidine, a weak nonsteroidal antiandrogenic drug, induces testis toxicity in male rats46 and widely used in the treatment of gastric and duodenal ulcers and in the symptomatic relief of other gastrointestinal disorders including gastroesophageal reflux disease, Zollinger ellison syndrome.47 In present study, cimetidine had no negative effect on the body and testicular weight after long time exposure by gastric gavage, this concur with the report of Xu et al.,48 that oral administration of cimetidine had no adverse influence on the body and relative weight of reproductive organs after 9 weeks of treatment. However, administration Ocimum gratissimum extract significantly decrease the body weight but had no significant influence on testicular weight.
In current study, cimetidine decrease sperm volume and motile sperm count, percentage motility, total count, fast progressivity and normal sperm morphology were significantly decrease in cimetidine treated group as compared with the control group treated with normal saline which is constant with the finds of Aprioku et al.,49 and Xu et al.,48 that sperm count and progressive sperm count had a decreasing trend and have adverse effects on the activation of spermatozoon motility but disagree that cimetidine did not cause any significant effect on sperm morphology and viability. Since sperm count is a vital property of sperm, male fecundity decreases progressively with reduction in sperm concentrations50 and sperm motility is a critical indicator of semen quality and fertility potential.51 Therefore, cimetidine cause impairment in seminiferous tubular function and alter normal spermatogenesis in rat.52 However, intervention of aqueous extract Ocimum gratissimum increased the motile sperm count, percentage motility, total count, fast progressivity and normal sperm morphology and ameliorate the toxic effect of cimetidine on sperm quality. Increase in sperm count of rats administered Ocimum gratissimum extract may be due to increase in testosterone production, since testosterone promotes spermatogenesis. Furthermore, increase in sperm motility could be due to increase in mitochondrial activity and fructose synthesis, as well as high ATP content.53 This disagree with the report of Shehu-Tijani et al.,54 that Ocimum gratissimum poses anti-fertility effects on normal and diabetic rats and Leigh and Fayemi55 also reported that aqueous extract of Ocimum gratissimum has deleterious effects on both spermatogenesis and maturation of spermatozoa at different stages of germ cell development. Cimetidine reduced FSH, LH and testosterone levels and elevate prolactin level when compared with the control group. Cimetidine decrease LH levels by antagonizing the feedback control of gonadotrophin secretion by androgens and desensitizes androgen receptors and inhibits the negative feedback for gonadotrophin secretion. Subsequent to increased LH levels, an elevation in testosterone levels occurs. Generally, FSH and LH are synthesized and secreted by gonadotropic cells in the anterior pituitary lobe are necessary for maintaining testosterone levels such that as their testicular concentration increases testosterone increases.56 Since testosterone is required for the growth and development of male reproductive organs and in association with FSH, acts on the seminiferous tubules to initiate and maintain spermatogenesis.57 Therefore, Serum FSH and LH level are adjusted by negative feedback.58 Increase in prolactin and decrease testosterone constant with the report of Pimpinellei et al.,59 that increased prolactin synthesis could directly inhibit testosterone synthesis. Treatment with Ocimum gratissimum regulate the hormone level.
In this study, sexual competence is interactions between nervous, endocrine, and vascular systems and their normal coordinated activities associated with sexual rejuvenation, intercourse and vigour. Normal sexual response cycle in males comprised 5 physiologically interrelated sequences that occur in a defined consecutive pattern (libido, erection, ejaculation, orgasm and detumescence), disruption in the sequence may lead to sexual dysfunction,60 administration of cimetidine in this study induced sexual dysfunction by significantly (p<0.05) decrease MF, IF, EF and EL and increased ML, IL and PEL However, administration of Ocimum gratissimum only and co administration of cimetidine with Ocimum gratissimum elevate MF, IF, EF and EL and reduced ML, IL and PEL. Mount and intromission frequencies are essential parameters of libido, vigour, and potency. While MF suggests sexual motivation, enhanced IF is reflective of coherent erection, penile orientation and the comfort by which ejaculatory reflexes are stimulated.61 Therefore, increases in MF and IF following administration Ocimum gratissimum indicative of improved libido36 as a result of elevated levels of anterior pituitary hormones and testosterone, which stimulated sexual competence through presence of plug in the vagina of the female rats and the genital toileting in the extract-treated male rats were indicative of ejaculation. Therefore, increase in EF by Ocimum gratissimum is an indication of improved aphrodisiac potential of Ocimum gratissimum. Whereas, MF and IF increased values are linked with sexual motivation in animals, those of ML and IL are inversely related.62 Therefore, the significantly decreased ML and IL values by Ocimum gratissimum could be informative of invigorated sexual motivation and enhanced competence. Moreover, the reversion of short-lived EL in cimetidine treated rats to significantly prolonged EL following treatment with Ocimum gratissimum shows that the extract composed of bioactive components responsible for lengthened coitus period and facilitated copulatory performance in animals. This was further supported by the display of pelvic thrusting during intromission and ejaculation by the Ocimum gratissimum treated rats. The PEL is an invaluable assessment tool for potency, libido and the rate of recovery from exhaustion after initial sets of mating.63 Thus, the significantly attenuated PEL in the Ocimum gratissimum treated rats could be linked to energy boost and less exhaustion in the initial sets of mating by the male rats. It may also be suggestive of enhanced potency and libido. Our submissions are consistent with the report of Watcho et al.,64 where significantly reduced PEL (in the range≤5400s) was considered optimum for sexual satisfaction.
In our observation, administration of cimetidine reduced the testicular enzymes (LDH, ACP, and ALP) activities in cimetidine treated group when compared with the normal control group. Cimetidine administration thus, inhibit the specific activities of ALP, ACP and LDH in the testes65 by prevents ejaculation and abolishes orgasm which evidence in our copulatory test. Testicular ALP and ACP are important enzymes involved in steroidogenesis channelizing materials for the process and maintenance of sperm quality.66 Hence, the reduced activities of these enzymes are associated with inhibitory role of cimetidine which resulted in reduction in steroidogenesis as well as overall decrease in libido and sexual competence as evidence in our observation. The significantly decreased testicular LDH activity in cimetidine treated rats suggests possible reduction in energy metabolism which obstructed the spermatogenesis by preventing transformation of spermatocyte to spermatozoa. Therefore, increase in testicular enzymes (LDH, ACP, and ALP) activities following treatment with aqueous extract of Ocimum gratissimum revealed protective and androgenic potentials of the Ocimum gratissimum extract.
Furthermore, there was significant decrease in testicular concentrations of protein, cholesterol and glycogen in cimetidine treated rats, the significant reduction in the concentration of testicular protein could be link to impaired spermatogenesis or defective sperm maturation process, the decrease in glycogen levels might be a manifestation of gradual and steady depletion of energy reserve that subsequently impacted on the motility aided energy metabolism in sperm cells of the rats, Similarly, the significantly reduced testicular cholesterol concentration in animals treated with cimetidine alone suggest altered and overall reduction in steroidogenesis particularly on the androgens. However, administration of Ocimum gratissimum and co administration of cimetidine and Ocimum gratissimum significantly increased concertation of testicular protein, cholesterol and glycogen when compared with cimetidine treated rats. Significant increase in concentration of testicular protein, cholesterol and glycogen enhanced spermatogenesis and sperm maturation. Spermatogenesis and subsequent maturation of sperm cells, steroidogenesis and steady supply of energy for sperm motility are linked to testicular levels of cholesterol and glycogen, respectively.67 Increased in testicular cholesterol concentration by Ocimum gratissimum is attributed to its improved moderation on androgen concentration which enhanced steroidogenesis.68
Histological examination of the testis shows that cimetidine caused severe degeneration of seminiferous epithelium with relatively wide empty lumen, seminiferous tubular atrophy with epithelial disorganization, reduction in the thickness of the germinal epithelium, vacuolization of spermatogonia, poor differentiation of spermatogenic germ cells, maturation rest and depressed spermatogenesis this support the results of previous finds.69,70 The testes showed suppression of spermatogenesis which resulting in oligospermia (reduced number of sperms), oligospermia may occur due to long treatment of cimetidine.71 Administration of Ocimum gratissimum aqueous leaf extract regenerate and maintained the his-toachitecture of the testis, increased the proliferative activity of spermatogonia and maintained the volume density of the interstitium compared to the control animals. From our observation, when Ocimum gratissimum aqueous leaf extract was administered simultaneously with cimetidine; it protected the testis from the deleterious effects of cimetidine. This protective property of Ocimum gratissimum is enhanced by some of its phytochemical constituents: the presence of ascorbic acid which is known for its protection on cell membranes and its scavenging effects on free radicals.72 Our observation concurs with the findings of Anoka et al.,73 that butanolic fractions of methanolic leaf extract of Ocimum gratissimum preserved the histoarchitecture of the testes against ethyl acetate but disagree with the previous report by Obianime et al.,74 and Shehu-Tijani et al.,54 that Ocimum gratissimum caused distortion and destruction of the architecture and structure of the testicular histology with varying degrees of edema within the interstitial cells in normal mice. Also, in this study, there was observe significant decrease in tubular diameter, perimeter, width, length, germinal height, cross-sectional area, number of profiles per unit area, numerical density and increase in lumen diameter of the tubules in animals treated with cimetidine only comparison with control. Reduced seminiferous tubular diameters, depleted germ cells and irregular small seminiferous tubules with Sertoli cells post-cimetidine administration were also reported by Franca et al.,13 Cimetidine has been reported to cause apoptosis to testicular germ cells and Sertoli cells.14 The resultant damage was also associated with upregulation of p53 expression. Elevation of p53 protein expression in response to DNA damage triggers either a transient cell cycle arrest or apoptosis.75 Intervention of Ocimum gratissimum extract shows remarkable improvement in the histo-morphometric parameters.
ConclusionsIn conclusion, cimetidine induced testicular toxicity by depleted the germinal epithelium and caused widening and hypocellularity of the interstitium. However, Ocimum gratissimum protected the cyto-architecture of the testis from the damaging effects of cimetidine and promote germinal epithelial growth therefore, Ocimum gratissimum leaves augments spermatogenesis and attenuates cimetidine-induced testicular toxicity associated with cimetidine through an antioxidant system of activities.
Ethical disclosuresThe experimental procedures were conducted in accordance with the NIH guidelines for the care and use of laboratory animals and in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Protection of human and animal subjectsThe authors declare that no experiments were performed on humans for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Authors’ contributionsSA contributed in the design and supervision of the practical part. SA, BO and OW contributed in the design, histopathological examination, analysis of results and drafting the manuscript. SA shared in the study design and data interpretation. SA, OW and OVO have a major contribution in the practical part, analysis and discussion of results. SA, BO and OVO contributed mainly in the data collection, practical part, revising and drafting the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participateThe experimental procedures were conducted in accordance with the NIH guidelines for the care and use of laboratory animals in line with guidelines of Department of Human Anatomy Federal University of Technology, Akure Nigeria.
Consent for publicationNot applicable.
Availability of data and materialsData will not be shared because they are completely included within the manuscript.
FundingThis work was conducted through collaboration between different research facilities in Department of Human Anatomy, Federal University of Technology, Akure Nigeria and Department of Anatomy, Ladoke Akintola University of Technology, Ogbomoso Nigeria. No specific financial support was received by the authors from any individual or cooperate body during this research.
Conflict of interestsThe authors declare that they have no competing interests.
The authors appreciate Omomoh Bernand in herbarium section of Centre for Research and Development (CERAD) of the Federal University of Technology, Akure, Nigeria for the plant identification and authentication.