A regular circadian rest-activity pattern is essential for optimal health. Any disruption or irregularity can lead to alterations in circadian rhythms, which in turn can trigger cardiometabolic illnesses. This review presents an approach to the literature on the circadian rest-activity pattern and its association with cardiometabolic illnesses.
MethodNarrative review.
ResultsCircadian rest-activity pattern alterations, such as lower stability, reduced robustness, and higher fragmentation of circadian rest-activity rhythms, may be detrimental to human health. Furthermore, objective methods such as wrist actigraphy, questionnaires, and sleep diaries, as well as “dim light melatonin onset” (DLMO), have been proposed as feasible methods to assess the circadian rest-activity pattern.
ConclusionIn medical practice, it is crucial to view the circadian rest-activity pattern as a clinical biomarker related to human health. Additionally, alterations in the circadian rest-activity pattern have been linked to cardiometabolic illnesses.
Un patrón circadiano regular de descanso-actividad es esencial para una salud óptima. Cualquier interrupción o irregularidad en este patrón puede provocar alteraciones en los ritmos circadianos, que a su vez pueden desencadenar enfermedades cardiometabólicas. Esta revisión presenta una aproximación a la literatura existente sobre el patrón circadiano de actividad-reposo y su asociación con las enfermedades cardiometabólicas.
MétodoRevisión narrativa.
ResultadosLas alteraciones del patrón circadiano de actividad-reposo, como una menor estabilidad, una menor robustez y una mayor fragmentación de los ritmos circadianos de actividad-reposo, pueden ser perjudiciales para la salud humana. Además, se han propuesto métodos objetivos como la actigrafía de muñeca, los cuestionarios y los diarios de sueño, así como el inicio de melatonina con luz tenue (DLMO, por sus siglas en inglés), como métodos factibles para evaluar el patrón circadiano de actividad-reposo.
ConclusionesEn la práctica médica, es crucial considerar el patrón circadiano de actividad-reposo como un biomarcador clínico relacionado con la salud humana. Además, las alteraciones del patrón circadiano de actividad-reposo se han relacionado con enfermedades cardiometabólicas.
Circadian rhythms are physiological adaptations that align with the 24-hour day/night cycle on Earth. They consist of two main components: oscillatory and evoked, which contribute to rhythmic outputs1. In anatomical terms, the central pacemaker of circadian rhythms, the suprachiasmatic nucleus (SCN), is located in the hypothalamus. At a molecular level, clock genes play a key role in regulating gene expression and coordinating various physiological activities throughout the day and night in all species. The suprachiasmatic nucleus is composed of two nuclei, each with approximately 10,000 neurons, located on either side of the third ventricle, just above the optic chiasm2.
The retinohypothalamic tract is the neuronal pathway that circadian rhythms use to synchronize in the human body. This pathway is a complex network that begins in specialized cells located in the retina, called melanopsin cells, which act as retinal photoreceptors within intrinsically photosensitive retinal ganglion cells (ipRGCs). These photoreceptors have the main function of capturing the stimulus light from the environment and sending this light information to the SCN2,3.
During the nuclear feedback loop, genes such as Period (Per) and Cryptochrome (Cry) are expressed at night because of Brain and muscle arnt-like protein 1 (BMAL1) and circadian locomotor output cycles kaput (CLOCK). Conversely, at the start of the day, the PER-CRY dimer translocates into the nucleus, causing BMAL1 and CLOCK to uncouple and inhibit their expression. At the end of the day, the degradation of PER and CRY enables BMAL1-CLOCK to rejoin, resulting in a rhythmic oscillation within a 24-hour period2–4.
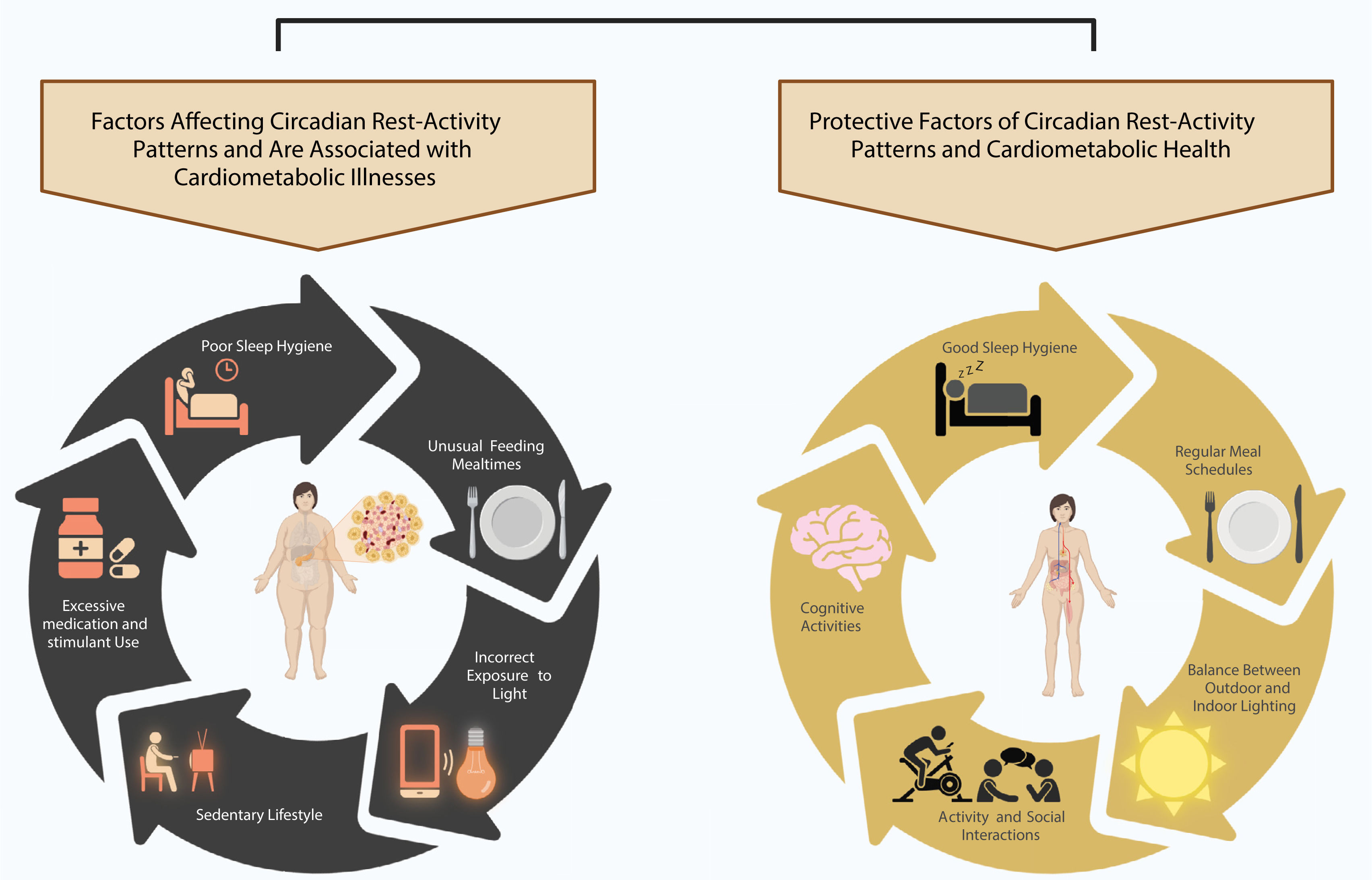
Furthermore, almost every tissue of the body has a peripheral clock, including the liver, pancreas, lung, and heart. The SCN transmits information to peripheral clocks through autonomic innervation, food cues, and extrinsic hormonal signals that regulate body temperature3. The balance between the central and peripheral circadian clocks is crucial for maintaining optimal health. Any deregulation of this balance can have a negative impact on human health, leading to the progression and/or worsening of cardiometabolic illnesses (Figure 1) such as type 2 diabetes mellitus (T2DM), high blood pressure (HBP), and dyslipidemia5–8. Several circadian rhythms have been extensively described in scientific literature, which include blood pressure, insulin, leptin, ghrelin, cerebral clearance, core and skin temperature, cortisol, melatonin, and circadian resting activity. These circadian rhythms are characterized by varying levels of plasma concentration, temperature, and fluid velocity throughout the 24-hour day/night cycle, fluctuating between the nadir and zenith8–12. In the case of circadian rhythm sleep-wake disorders, there is a persistent or recurrent pattern of sleep disturbance due to alterations in the circadian timing system or a misalignment between the individual's endogenous circadian rhythm and exogenous factors that affect the timing, duration, and quality of sleep. According to the International Classification of Sleep Disorders Third Edition (ICSD-3), seven circadian rhythm sleep-wake disorders have been described, and their diagnostic criteria are listed in Table 1.
Circadian rhythm sleep-wake disorders and Diagnostic Criteria According to International Classification of Sleep Disorders, Third Edition (ICSD-3).
| Circadian rhythm sleep-wake disorders | Diagnostic Criteria |
|---|---|
| • Delayed sleep-wake phase disorder (DSWPD)• Advanced sleep-wake phase disorder (ASWPD)• Irregular sleep-wake rhythm disorder (ISWRD)• Non-24-hr sleep-wake rhythm disorder (N24SWD)• Shift work disorder (SWD)*• Jet lag disorder (JLD)*• Circadian sleep-wake disorder, not otherwise specified. | 1- A chronic or recurrent pattern of sleep-wake rhythm disruption primarily due to alteration of the endogenous circadian timing system or misalignment between the endogenous circadian rhythm and the sleep-wake schedule desired or required by an individual's physical environment or social/work schedules.2- The circadian rhythm disruption leads to insomnia symptoms, excessive sleepiness, or both.3- The sleep and wake disturbances cause clinically significant distress or impairment in mental, physical, social, occupational, educational, or other important areas of functioning |
The sleep-wake cycle consists of non-rapid eye movement (non-REM), rapid eye movement (REM), and wakefulness stages. The non-REM phase is architecturally composed of three stages: N1, N2, and N3. During this phase, polysomnography (PSG) records low electroencephalographic signals characterized by low frequency (0.5 - 4Hz) and high amplitude delta oscillations13. In contrast, the REM phase is mainly characterized by theta (6 - 9Hz) and gamma (30 - 300Hz) frequencies. Similarly, during the electromyogram (EMG) test, non-REM is characterized by non-musculoskeletal ocular activity, in contrast to what is observed during the REM phase13. Furthermore, during the REM phase, vital signs become more erratic, displaying irregularities in blood pressure, breathing, and heart rate, as well as an increase in apneas and hypopneas14. The intensity of sleep and homeostasis is regulated by a cascade of events, which are promoted by neurotransmitters such as noradrenergic (NA), cholinergic, histaminergic, dopaminergic, and serotoninergic neurons that stimulate wakefulness. Conversely, other substances such as gamma-aminobutyric acid (GABA) and galanin act as inhibitors. From an anatomical perspective, sleep homeostasis primarily occurs in the forebrain, whereas the timing of sleep is regulated by the SCN in the hypothalamus13,15. In 2022, the American Heart Association (AHA) recognized sleep as an essential component of cardiovascular and brain health. Sleep duration has been linked to cardiovascular health, with a suggested duration of 7-9hours per night16. However, several studies report a higher prevalence of sleep alterations worldwide. Currently, there is evidence of an association between short sleep duration (=6h/night) and long sleep duration (≥9h/night) and all-cause mortality and cardiovascular risk in patients17.
Circadian rest-activity pattern and cardiometabolic illnessesType 2 Diabetes Mellitus (T2DM)Circadian rhythms are crucial for glucose metabolism. Any disruption or irregularity may lead to glucose intolerance, insulin resistance, and weight gain. In healthy individuals, glucose tolerance is higher in the morning than in the evening. This time-dependent glucose tolerance is regulated by the rhythmicity of pancreatic β cells. However, environmental factors can disrupt this balance of rhythmicity.
Sleep-wake schedules and food intake strongly influence this balance and may disrupt circadian rhythms. Clinical trials have shown that these disruptions can have deleterious metabolic effects on healthy populations9–11. For instance, a clinical trial by Grant, et al showed that nocturnal food intake in healthy men impaired glucose metabolism, increasing glucose levels by up to 70%10. Moreover, abnormalities in circadian rest-activity rhythms, such as higher fragmentation of the rest-activity rhythm within each 24-hour period, increase the risk of cardiometabolic illnesses and mortality in people with T2DM18,19.
High blood pressure (HBP)Blood pressure is dependent on a circadian rhythm. Prior to the end of the sleep period, there is a small increase in blood pressure, followed by a more pronounced rise 2-3hours after waking up. The rise is then even more pronounced near the middle or end of the activity period. During the night, blood pressure usually decreases by 10-20%, which is known as the normal dipper pattern. If the decrease is over 20%, it is called the extreme dipper pattern, while a decrease of less than 10% is referred to as the non-dipper pattern20.
The risk of cardiometabolic illnesses is closely related to respiratory and cardiovascular dysfunction including pulmonary hypertension and right ventricular dysfunction21. Moreover, patients with cardiometabolic illnesses under clinical control have shown a decrease of almost 50% in the reduction of cardiovascular disease (CVD) events22. Furthermore, in patients, HBP is often associated with DM, hypercholesterolemia, and obstructive sleep apnea (OSA)23.
OSA is frequently linked with HBP, suggesting a recurrent co-occurrence of these two conditions24, with an association observed both cross-sectionally and longitudinally25. In fact, REM sleep OSA is associated with the presence of non-dipping nocturnal systolic and diastolic blood pressure26.
During the REM stage, the stability of vital signs fluctuates; heart rate, respiratory rate, and blood pressure become irregular14. This fluctuating pattern may increase the risk of nocturnal cardiovascular events, especially in OSA patients. OSA is an independent risk factor for stroke, particularly in males. It is worth noting that OSA is more prevalent in males and that male sex and older age are considered risk factors for OSA. In this context, one of the primary features of OSA is the intermittent hypoxemia that occurs during sleep. Apnea mediated ischemia is one of the leading causes of cerebral isquemia27. Considering these aspects, ambulatory blood pressure monitoring should be conducted regularly throughout the day (24hours), particularly in patients who are suspected of having OSA28. In this sense, early diagnosis of both hypertension and OSA is crucial in the prevention of major cardiovascular events. In this way, prompt treatment such as continuous positive airway pressure (CPAP) could reduce the risk of recurrent major adverse cardiac and cerebrovascular events, suggesting that adherence to CPAP is a pivotal factor in secondary cardiovascular prevention in patients with OSA29.
StrokeStroke is one of the main causes of death worldwide30. According to the American Heart Association, 3.4 million US people aged =18 years will have had a stroke by 2030, representing a 20.5% increase in prevalence compared to 2012. Moreover, white Hispanic men are estimated to experience the highest increase of 29%23. Currently, cardiovascular risk and mortality are associated with short sleep duration (≤6h/night) and long sleep duration (≥9h/night), in contrast to the 7-8 hour recommendation for optimal health in the adult population17. A study by Huang T et al concluded that an increased risk of CVD was associated with greater diurnal variation in sleep duration and timing31. Additionally, the risk for CVD was more than twice as high in the less regular sleepers than in the more regular sleepers over the 4.9 years they were followed31.
During sleep, various physiological processes occur, including significant cerebral activity. One of the main processes that occurs during sleep is clearance, which involves the elimination of brain metabolites such as CO2, lactate, and proteins, including amyloid-β and tau proteins, which are closely related to Alzheimer's disease (AD)32. Regarding the balance of blood coagulation, both the coagulation and fibrinolytic systems exhibit circadian variability, which is characterized by prothrombotic and hypofibrinolytic states during the early morning hours. During this period, increased platelet aggregation and variations in blood viscosity have been reported33. Additionally, OSA patients have been found to exhibit increased blood fibrinogen and platelet aggregation activity during the first hours of the morning27.
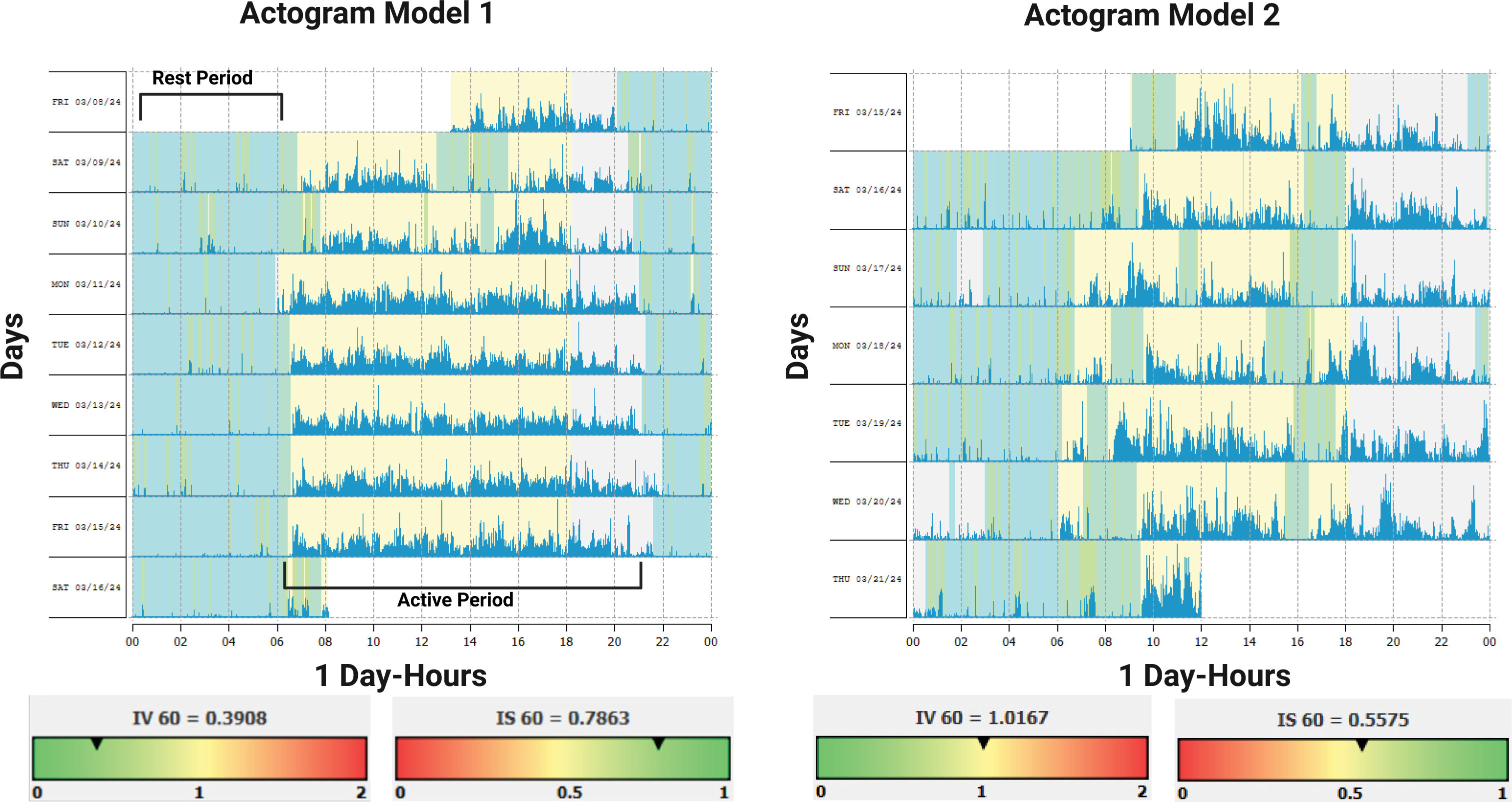
Clinical variables of the Circadian rest-activity rhythmAs it is known, luminal information is the most powerful synchronizer, obtained principally from natural sources such as sunlight. This luminal cue serves as marker of several circadian physiological processes in the human body allowing an optimal synchronized release of several hormones related to sleep and wakefulness, such as melatonin and cortisol respectively. In addition to luminal cues, physical activities, social behavior, and nutritional characteristics play a strong role as synchronizers. A regular circadian rest-activity pattern is essential for optimal health (Figure 2). At present, the non-parametric variables such as interdaily stability (IS), intradaily variability (IV), relative amplitude (RA), L5, and M10 have been suggested as novel biomarkers of cardiometabolic health and indicators of major post-stroke adverse outcomes19,34. The IS indicates the synchronization of the internal rest-activity rhythm with the different zeitgebers35. The stability of the rhythm is influenced by exposure to the light-dark cycle, the integrity of the retina/retinohypothalamic tract system, the presence of social synchronizers, and physical activity36; whereas the intradaily variability reflects the fragmentation of the rest-activity rhythm within each 24-hour period37. The RA parameter reflects the difference between the mean activity of the 5 consecutive hours with the lowest activity (L5) and the mean activity of the 10 consecutive hours with the highest activity (M10). A high magnitude indicates a well-defined or healthy rhythm; therefore, a higher RA represents a stronger circadian rhythm35,36,38.
Actograms models, normal and abnormal rest- activity pattern
Abreviations: Interdaily Variability (IV); Intradaily Stability (IS). Figure 1 shows two actogram models. The light blue rectangles represent the rest period, while the blue signals represent the active period. Model I demonstrates a Circadian Rest-Activity Pattern that exhibits expected levels of fragmentation (IV) stability (IS), and amplitude. Model 2 demonstrates a high fragmentation (IV) of the Circadian Rest-Activity Pattern, with periods of inactivity during the day and/or periods of activity during the night. Additionally, the stability(IS) of the rest-activity rhythm throughout the daily period is low with respect to the time of sleeping and waking.
Created with BioRender.com.
In addition, melatonin is the major hormone involved in regulating the cycle between sleep and wakefulness. It is synthesized within the pinealocytes from tryptophan, mostly during the dark phase of the day. The production of melatonin follows an internally generated rhythm that is controlled by interacting networks of clock genes in the bilateral SCN. In humans, light can affect melatonin production. For instance, research has demonstrated that exposure to 2 500 lux of light at night completely suppresses melatonin production, while exposure to less than 200 lux can still suppress secretion and lead to changes in circadian rhythms39. In this line, the melatonin levels of the adult population peak at 02:00 and 04:00hours averaging about 60 to 70 pg/ml. In clinical and research contexts, it is crucial to use dim light melatonin onset (DLMO) as a reliable marker of circadian phase position and; therefore, as a valuable tool for understanding circadian rhythms40.
Circadian rest-activity patterns alterationsThe actigraphy device enables the recording of several basic variables that indicate sleep patterns and daily rhythms36. Specifically, the wrist actigraphy device is a valuable tool for obtaining information on sleep and circadian rhythms from motor activity36. Currently, it has been demonstrated that human wrist activity exhibits a robust circadian pattern. This pattern can be evaluated objectively using an actigraphy device to assess the robustness of the circadian rest-activity rhythm41. Greater rhythmic stability (IS), lower fragmentation (IV), and a robust rhythm (RA), characterized by an active wake period (M10) and an earlier and more restful sleep period (L5) are associated with a lower prevalence of hypertension, obesity, and central obesity5.
Several studies have shown the correlation between circadian rest-activity rhythms alterations and cardiometabolic illnesses6,8,12,19. Thus, in patients with T2DM, alterations in circadian resting-state activity rhythms may increase the risk of CVD and mortality, and are associated with ischemic heart disease (IHD) and greater mortality from all causes18,34. Recently, a study by Yang et al18, suggests abnormalities in circadian rest-activity rhythms in patients with DM including low IS and low RA. In addition, this study concluded that higher IV and low RA were also significantly associated with CVD mortality. Likewise, the findings of Yeung et al7 suggest a correlation between disrupted rest-activity rhythms and higher odds of HBP7. Similarly, Gao et al42 conducted a study to evaluate the association between 24-hour rest-activity rhythm measures, stroke risk, and major post-stroke adverse outcomes. The study suggested an association between lower M10 and higher L5 (suppressed RA) and stroke risk. Additionally, a more fragmented rhythm was associated with a higher risk of stroke42. These results were independent of sociodemographic variables and comorbidities including obesity, sleep disorders, and CVD. Furthermore, Cespedes-Feliciano et al12, concluded that lower RA or less robust circadian rest-activity patterns were independently associated with higher BMI, further suggesting that the strength of the circadian rest-activity pattern may influence energy balance independently of TST and activity.
Circadian rest-activity rhythm in critical survivors and Alzheimer's diseaseThe intensive care unit (ICU) environment has the potential to sustain life. During the patient's stay in the ICU, the physiological changes related to their admission diagnosis, as well as the ICU environment, can disrupt their circadian rest-activity patterns43. The use of drugs, medical procedures, invasive mechanical ventilators (IMV), and alarm noises can contribute to these disruptions. Similarly, irregular feeding schedules, lack of social contact, a significant decrease in motor activity, and light exposure can disrupt circadian rhythms43, which are synchronized by these factors.
ICU survivors of COVID-19 may experience alterations in their circadian rest-activity pattern, including increased fragmentation and reduced robustness. Benitez et al concluded that survivors of critical COVID-19 may experience poor sleep quality and alterations in their circadian rest-activity pattern three months after ICU discharge35. Similar findings were reported four months after ICU discharge, with a higher prevalence of sleep and circadian rest-activity pattern disturbances observed in survivors of critical COVID-1944. In the study conducted by Targa et al, the authors found that sleep and circadian disturbances persisted in COVID-19 survivors even 6 months after being discharged from the ICU45. In this context, various therapeutic interventions on circadian rhythms during and after intensive care have been proposed to prevent and reduce the adverse effects on pre-existing illnesses or avoid their ocurrence.
Aging is associated with less robust circadian rhythms and reduced circadian function, suggesting that circadian dysfunction may promote neurodegeneration and eventually be involved in the pathogenesis of Alzheimer's disease (AD)46. Higher levels of inter-day variability have been found in Alzheimer's patients, as well as associations between inter-day variability and poorer sleep quality, lower rest-activity rhythm amplitude, and poorer cognitive and motor performance36.
Targa et al47, found similar associations, suggesting that older age is associated with increased rhythm fragmentation. In addition, this study suggests that male individuals are associated with worse circadian resting activity pattern findings. Similar evidence was observed by Musiek et al46, which concludes that preclinical AD is associated with rest-activity rhythm fragmentation, regardless of age or sex. Furthermore, Musiek et al suggest that preclinical circadian rest-activity alterations in AD may contribute to early disease pathogenesis or serve as a biomarker of preclinical disease.
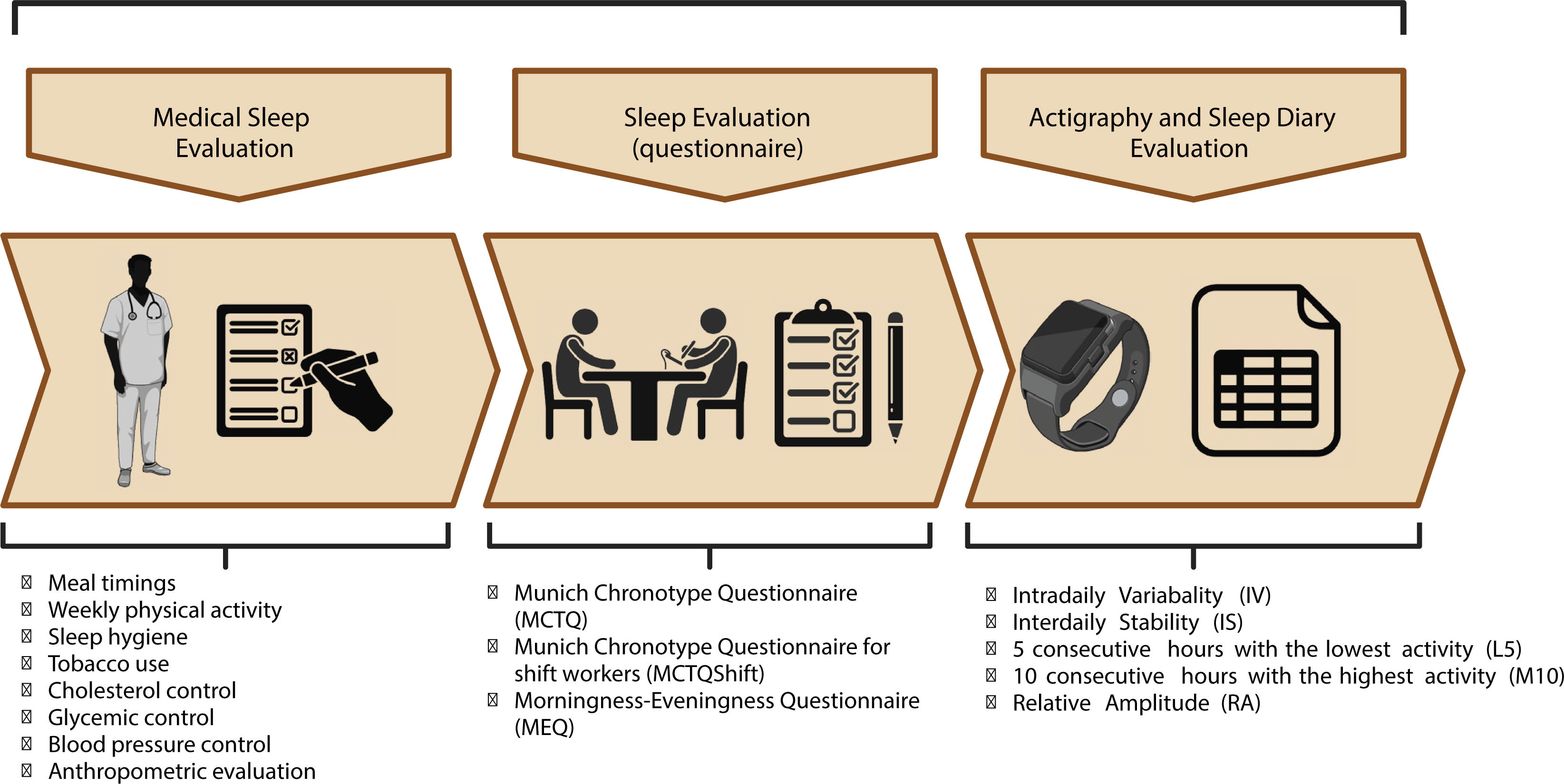
Analysis of circadian rest-activity patterns in clinical practiceIn clinical practice, it is crucial to consider a variety of factors and to have useful tools to analyze circadian patterns of rest and activity (Figure 3). Sleep diaries or sleep logs are useful tools for measuring sleep timing and circadian rhythm sleep-wake disorders. It is important to note that these methods are subjective and should be clearly marked as such. They have several advantages in a clinical context, including being low-cost, noninvasive, and easy to use48. However, the use of these tools in the absence of actigraphy may lead to an overestimation of sleep-wake patterns. It has been suggested that 7 days of actigraphy wrist monitoring accompanied by sleep diaries can provide more accurate results. The combined use of actigraphy and sleep diaries has been found to be correlated with PSG49.
Chronotype refers to an individual's resting and activity pattern preferences and is closely associated with the circadian system. Different questionnaires have been proposed to determine chronotypes in populations. The Morningness-Eveningness Questionnaire (MEQ) is a valuable tool for indicating an individual's chronotype. The questionnaire categorizes chronotypes as Extreme Evening, Moderate Evening, Intermediate, Morning Moderate, and Extreme Morning4.
In the occupational context, shift workers are at the highest risk of developing circadian misalignment and cardiometabolic illnesses50–52. Shift work disorders are characterized by sleep disturbances related to working hours that overlap with usual sleep times53. Clinicians should consider assessing the chronotype, particularly in shift workers, when there is suspicion of circadian rhythm misalignment. However, this is often ignored in work settings, and strategies to mitigate the effects of shift work are often lacking.
In addition, the Munich Chronotype Questionnaire (MCTQ) is a validated questionnaire that serves as a self-rated scale to assess individual phases of entrainment on work and work-free days. It was developed for ages 6 to over 65 years. Similar to the MEQ, the MCTQ categorizes chronotype as follows: 0=extreme early, 1=moderate early, 2=slight early, 3=normal, 4=slight late, 5=moderate late, and 6=extreme late54. Furthermore, in shift workers, the Munich Chronotype Questionnaire for Shift Workers (MCTQShift) has been proposed as a useful instrument to determine the chronotype of this population55. In summary, research has shown that shift work, sleep disturbance, and disruption of circadian rhythms are strongly associated with cardiometabolic stress1.
ConclusionRecent studies have shown a close association between circadian rest-activity patterns and cardiometabolic illnesses. This suggests that circadian rest-activity patterns could be relevant biomarkers for human health. In a clinical context, considering circadian resting activity patterns as a biomarker could be useful for assessing the trajectory during treatment of cardiometabolic illnesses, as well as adherence to treatment and short and long-term treatment outcomes. Finally, incorporating circadian rest-activity patterns can help to identify risk factors that may worsen cardiometabolic illnesses and subsequently promote protective health factors or healthy lifestyles.
Conflicts of interestThe authors have no conflict of interest to disclose.