To examine the effectiveness of playing chess as a treatment option for children with ADHD.
MethodsParents of 44 children ages 6–17 with a primary diagnosis of ADHD consented to take part in the study. Parents completed the Spanish version of the Swanson, Nolan and Pelham Scale for parents (SNAP-IV) and the Abbreviated Conner's Rating Scales for parents (CPRS-HI) prior to an 11-week chess-training program. We used a paired t-test to compare pre- and post-intervention outcomes, and Cohen-d calculations to measure the magnitude of the effect. The statistical significance was set at p<.05.
ResultsChildren with ADHD improved in both the SNAP-IV (t=6.23; degrees of freedom (df)=41; p<.001) and the CPRS-HI (t=5.39; df=33; p<.001). Our results suggest a large effect in decreasing the severity of ADHD as measured by the SNAP-IV (d=0.85) and the CPRS-HI (d=0.85). Furthermore, we found a correlation between intelligence quotient and SNAP-IV improvement (p<.05).
ConclusionsThe results of our pilot study should be interpreted with caution. This pilot project highlights the importance of carrying out larger studies with a case–control design. If our results are replicated in better designed studies, playing chess could be included within the multimodal treatment of ADHD.
Examinar la efectividad del juego de ajedrez como opción de tratamiento en niños con TDAH.
MétodosLos padres de 44 niños en edades comprendidas entre 6 y 17 años, con diagnóstico primario de TDAH, dieron su consentimiento para participar en el estudio. Los padres completaron la versión española de la Escala de Swanson, Nolan y Pelham para padres (SNAP-IV) y las Escalas Abreviadas de Puntuación de Conner (CPRS-HI), con anterioridad a un programa de entrenamiento ajedrecista de 11 semanas de duración. Utilizamos la t de student pareada para comparar los resultados previos y posteriores a la intervención, así como la d de Cohen para medir la magnitud del efecto. La significación estadística se estableció en p<0,05.
ResultadosLos niños con TDAH reflejaron una mejoría tanto en la escala SNAP-IV (t=6,23; grados de libertad (gl)=41; p<0,001) como en la CPRS-HI (t=5,39; gl=33; p<0,001). Nuestros resultados evidencian un elevado tamaño efecto en la disminución de la severidad del TDAH, según las mediciones de SNAP-IV (d=0,85) y CPRS-HI (d=0,85). Además, hallamos una correlación entre el cociente de inteligencia y la mejoría de la escala SNAP-IV (p<0,05).
ConclusionesLos resultados de nuestro estudio piloto deberán interpretarse con cautela. Este proyecto piloto subraya la importancia de realizar estudios más amplios con un diseño de control de casos. De replicarse nuestros resultados en unos estudios mejor diseñados, el juego del ajedrez podría incluirse en el tratamiento multimodal del TDAH.
Attention deficit hyperactivity disorder (ADHD) is a major public health issue.1 ADHD is the most frequent psychiatric disorder diagnosed during childhood and adolescent, affecting 4–8% of children worldwide.2 It is considered a chronic disorder with a substantial economic burden, estimated to be up to US$ 52.4 billion annually.3 In the diagnostic and Statistical Manual of Mental Disorders (DSM-5), age of onset criterion for the classical triad of ADHD charac;teristics–inattention, hyperactivity, and impulsivity–previously established at seven in the Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (DSM-IV),4 has been raised to 12.5 These features must persist 6 months or more, generate impairments across two settings or more (i.e. home, school), and are not better explained by another mental disorder. ADHD is frequently comorbid,6 and is associated with adverse outcomes including social exclusion, substance use, or even criminality.7 Thus, if ADHD remains untreated, children may not achieve their full potential in adulthood.8
A multimodal treatment is the most effective treatment of ADHD.8 The multimodal approach normally includes pharmacological treatments (i.e. metylphenidate, lisdexamfetamine, atomoxetine), psychological treatment (i.e. cognitive behavior therapy, family therapy), and psychoeducation. Pharmacological intervention is the treatment of choice in children and adolescents with severe ADHD,9,10 and the most common and convenient treatment for ADHD in developed countries.11 Unfortunately, many parents are reticent to putting their child on pharmacological treatment that may provoke side effects, such as insomnia, appetite suppression, or growth retardation.12 In addition, around 30% of ADHD children do not respond to these stimulants.13 Additionally, psychotherapy can be expensive.14 Thus, in this context, testing the putative therapeutic effect of chess training (“chess therapy”), a thousand-year-old board game that may improve attention and concentration, is not only interesting but worthwhile.15,16
Although chess is a traditional board game based on very simple rules, it requires the use of complex cognitive strategies. It has been used previously as a therapy option; for example, to enhance cognitive abilities in schizophrenia,17 and to prevent dementia.18 Given its educational benefits, the European Parliament recently made a declaration to encourage the introduction of the program ‘Chess in School’ into the educational systems of the European Union.19 To the best of our knowledge, however, there are no empirical studies testing if chess could be a psychotherapeutic alternative for ADHD. This is surprising given that central executive dysfunction is core to ADHD,20 and several executive functions are needed when playing chess.21 However, it is important to stress that executive dysfunction is neither necessary nor sufficient to cause ADHD.22
Given the limited evidence supporting the use of chess as a therapy for ADHD, we devised a pilot program with chess experts using chess training. The aim of the present pilot study was to test if a three month chess training course decreases ADHD severity in a naturalistic sample of de novo (recent diagnosis; medication-naïve) and diagnosed, pharmacologically treated ADHD children. We hypothesized that children who spent more time playing chess or children with higher intelligence were most likely to display improvements of ADHD symptoms.
MethodsParticipantsThis 11 week pilot non-comparative descriptive study was carried out at the Villalba Mental Health Center (Madrid, Spain). Forty-four children between the ages of 6 and 17, with a primary diagnosis of ADHD attending our facilities, entered into the study. Inclusion criteria included being 6–17 years old, and presenting with either a previous or de novo diagnosis of ADHD (DSM-IV). Exclusion criteria were sensorimotor handicaps (i.e. blindness, deafness), major neurological diseases (i.e. epilepsy), psychosis, mental retardation, and generalized developmental disorders.
Children who met criteria for current ADHD (DSM-IV) were offered to take part in the Jaque Mate al TDAH (Check mate of ADHD) project. The diagnosis was based on clinical interviews with each child and at least one parent carried out by a child psychiatrist. After the initial diagnostic interview, all participants and their parents/caregivers were evaluated using a semi-structured interview by a pediatric nurse. We built an ad hoc protocol including sociodemographic characteristics, past clinical history, various scales (see setting), and some school and chess training parameters (i.e. number of hours played every week, and assistance to chess lessons).
SettingThe study consisted of an 11-week trial of chess training. All children had weekly 1h sessions over a period of 11 consecutive weeks taught by a chess expert. ADHD children and adolescents took chess training lessons in groups of up to 10 individuals. Additional practice was suggested at home.
Pre- and post-program measuresThe severity of ADHD was evaluated using the Spanish version of the Swanson, Nolan and Pelham Scale for parents (SNAP-IV),23 and the Abbreviated Conners Rating Scales for parents (CPRS-HI)24 at each visit (baseline, T1; endpoint, 11 weeks±2 weeks, T2). The SNAP-IV is an 18-item checklist scored in a 4-point Likert scale ranging from Not at All (0) to Very Much (3) [range 0–54]. This scale is one of the most frequently used instruments to evaluate the response to treatment. For instance, the SNAP-IV was used in the NIMH Collaborative Multisite Multimodal Treatment Study of Children With Attention-Deficit/Hyperactivity Disorder (MTA).25–27 We used raw scores for comparison of improvement. The CPRS-HI is a 10-item, well-validated screening instrument for ADHD.24 This instrument consists of 10 behavior statements rated on a 4-point Likert scale with a total score ranging from 0 to 30.24 The scale was obtained from the longer versions of the Conners scales.28 A score of 15 or higher has been considered to be the clinically meaningful screening diagnostic cut-off point for diagnosing children with hyperactivity.29,30
Parents were blind to their previous ratings in both scales during each evaluation.
Data analysesMeans and standard deviations (SD) were calculated for the variables considered in our study. We used a paired t-test to compare variables pre- (Time 1; T1), and post- (Time 2; T2) treatment. Within subject change scores were calculated from the difference in pre- to post-treatment scores (i.e. T2 to T1). In addition, within group effect sizes, defined as the difference between the pre- and post-treatment intervention mean divided by the mean SD, were calculated using Cohen's d statistic for each scale (Cohen, 1988). For practical purposes, we used the effect size calculator at: http://www.uccs.edu/∼lbecker/. A positive effect size was considered as a measure of improvement (decrease in ADHD severity). The cutoff point for considering statistical significance was set at p<.05. We used SPSS for Macintosh Version 20 (SPSS Inc., Chicago, USA) for all the analyses.
EthicsAfter a complete description of the study, all children and their parents signed the written consent. The study was approved by the Puerta de Hierro Hospital Institutional Review Board (IRB) committee. All participants received a diploma for participating at the end of the study.
ResultsParticipant characteristicsForty-four children and adolescents were sequentially included. Reasons for not entering into the study were either because parents lived too far away to drive their children on a weekly basis or because the children did not enjoy chess playing (n=8).
Tables 1 and 2 display participant characteristics. Mean age (SD) was 10.73 years (±2.24). Most participants were male (70.5%). The mean number of children per classroom was 21±6.5. Nearly 90% of the participants were receiving additional teaching support at school, thus suggesting that our sample was particularly impaired with regard to school performance. The majority (81.8%) of participants had ADHD, combined type, and 61.4% were taking medication for ADHD. Also, a relevant percentage (40%) had at least another mental disorder (DSM-IV).
Participant sociodemographics.
| Variable | n (%) |
|---|---|
| Gender (male) | 31 (70.5) |
| Immigrant | 7 (15.9) |
| Adoption | 4 (9.1) |
| Ethnicity | |
| Caucasian | 35 (79.5) |
| Gipsies | 1 (2.3) |
| Asian | 1 (2.3) |
| Other (mulatto, maghrebi) | 4 (9.1) |
| Living with siblings | |
| No | 11 (25) |
| With biological siblings | 29 (65.9) |
| With non-biological siblings/half-siblings | 3 (6.8) |
| Position within siblings | |
| Only child | 8 (18.2) |
| Older brother/sister | 13 (29.5) |
| Second | 13 (29.5) |
| Non-identical twins | 2 (4.5) |
| Monthly family income | |
| <500€ | 8 (18.2) |
| 500–1499€ | 5 (11.4) |
| 1500–2000€ | 10 (22.7) |
| 2001–2500€ | 6 (13.6) |
| >2500€ | 12 (27.3) |
Participant school and clinical characteristics.
| Variable | n (%) |
|---|---|
| Type of school | |
| Public | 22 (50) |
| State-subsidised | 13 (29.5) |
| Private | 1 (2.3) |
| Education level | |
| Primary | 33 (75) |
| Secondary | 10 (22.7) |
| Repeating a school year (% yes) | 17 (38.6) |
| Receiving additional teaching support at school (% yes) | 38 (86.4) |
| Receiving additional teaching support at home (% yes) | 22 (50) |
| Special education needs (% yes) | 4 (9.1) |
| Adapting early childhood curriculum (% yes) | 11 (25) |
| After-school programs (% yes) | 17 (38.6) |
| Intelligence coefficient (% available) | 18 (40.9) |
| 70–79 (borderline deficiency) | 2 (4.5) |
| 80–89 (dullness) | 1 (2.3) |
| 90–109 (average/normal) | 10 (22.7) |
| 110–119 (superior intelligence) | 4 (9.1) |
| 130–139 (gifted) | 1 (2.3) |
| Current ADHD subtype | |
| Combined type | 36 (81.8) |
| Predominantly inattentive type | 8 (18.2) |
| Current DSM-IV psychiatric comorbidity (% yes) | 18 (40.9) |
| Learning disorders | 4 (9.1) |
| Communication disorders | 1 (2.3) |
| Conduct disorder | 4 (9.1) |
| Oppositional defiant disorder | 2 (4.5) |
| Elimination disorders | 1 (2.3) |
| Attachment disorders | 4 (9.1) |
| Major depression | 2 (4.5) |
| Medication for ADHD (% yes) | 27 (61.4) |
| Metylphenidate (different formulations) | 24 (54.1) |
| Atomoxetine | 3 (6.8) |
Forty-two participants (95.4%) completed the study, indicating an elevated adherence rate.
Two children dropped out after two chess training sessions. As might be expected, no adverse events were reported in any case. In one case, a 6-year-old girl on concerta® 18mg, supplementation with risperidone (1mg per night) was necessary to control her elevated impulsiveness and conduct disorder; no other changes in the treatment regimen were necessary in the remaining participants during the study.
On average, children attended 6 of the 11 sessions (6.25±2.02) with a range of 2–11. Children reported an average of nearly two hours per week (1.71±1.53) of chess training at home. De novo ADHD children did not practice significantly more hours than pharmacologically treated children (χ2=0.127; df=1; ns).
Exploratory pre- and post-treatment assessment35 of the 42 (83%) and 28 out of 34 (82%) parents in the T2 assessment reported a reduction in the SNAP-IV and the CPRS-HI, respectively. Following the convention used in pharmacological trials, a clinically significant decrease in ADHD severity was defined as a reduction of 30% or more in either the SNAP-IV or the CPRS-HI score. Thus, 16 of the 42 (38%) and 16 of 34 (47%) parents reported at least a 30% ADHD severity reduction in the SNAP-IV and CPRS-HI, respectively (57% of parents in at least one of these scales).
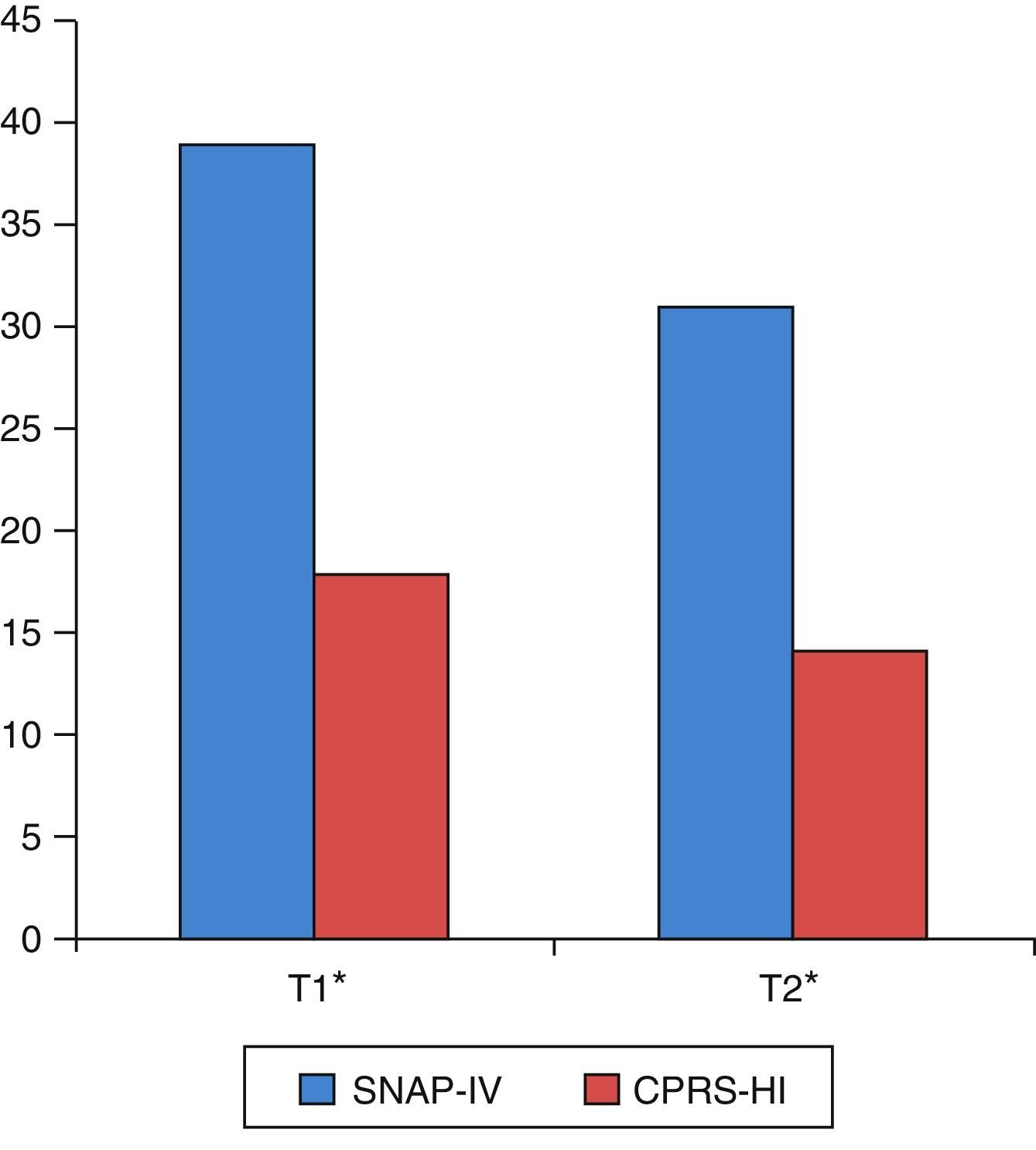
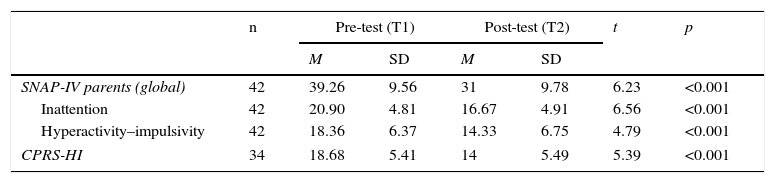
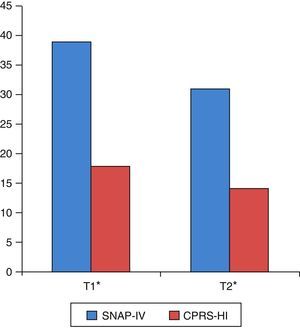
As shown in Table 3 and Fig. 1, there were statistically significant pre- to post-training improvements in ADHD severity as measured by both SNAP-IV and CPRS-HI.
Pre- and post-training changes in the SNAP-IV and the CPRS-HI.
| n | Pre-test (T1) | Post-test (T2) | t | p | |||
|---|---|---|---|---|---|---|---|
| M | SD | M | SD | ||||
| SNAP-IV parents (global) | 42 | 39.26 | 9.56 | 31 | 9.78 | 6.23 | <0.001 |
| Inattention | 42 | 20.90 | 4.81 | 16.67 | 4.91 | 6.56 | <0.001 |
| Hyperactivity–impulsivity | 42 | 18.36 | 6.37 | 14.33 | 6.75 | 4.79 | <0.001 |
| CPRS-HI | 34 | 18.68 | 5.41 | 14 | 5.49 | 5.39 | <0.001 |
We repeated these analyses separating by gender. In males (n=30), both the SNAP-IV and CPRS-HI significantly improved (40±9.63 vs 31.4±10.48, p<.001; and 19.54±5.47 vs 14.92±5.91, p<.05, respectively). In females (n=12), however, the SNAP-IV improved after chess training (37.42±9.53 vs 30±8.1, p<.05), while this was not the case for the CPRS-HI (16.60±4.90 vs 11.80±3.70, ns).
Additionally, we split our sample into de novo (n=17) and pharmacologically treated children (n=25). In de novo ADHD children, the SNAP-IV significantly improved (38±9.91 vs 30.70±10.67; p<.001) whereas the differences in the CPRS-HI between pre- and post-chess training did not reach statistical significance (17.73±5.45 vs 14±5.74; ns). In pharmacologically treated children, both the SNAP-IV and CPRS-HI significantly improved (40.12±9.42 vs 31.20±9.34, p<.05; and 19.42±5.4 vs 14±5.45, p<.001, respectively).
Also, we split our sample into patients with comorbidity (n=17) and without comorbidity (n=25). In ADHD children with comorbidity, both the SNAP-IV and CPRS-HI improved, but did not reach statistical significance in the CPRS-HI (38.76±9.82 vs 31.4±9.28, p=.001; and 19.21±4.45 vs 14.35±5.15, ns, respectively). As for ADHD children without comorbidity, the SNAP-IV significantly improved (39.6±9.56 vs 30.72±10.28; p=.006); the differences in the CPRS-HI between pre- and post-chess training were also statistically significant (18.3±6.1 vs 13.75±5.83; p<.001).
In order to test the magnitude of the difference between pre- and post-intervention, we calculated effect sizes for the SNAP-IV (d=.85) and the CPRS-HI (d=.85).
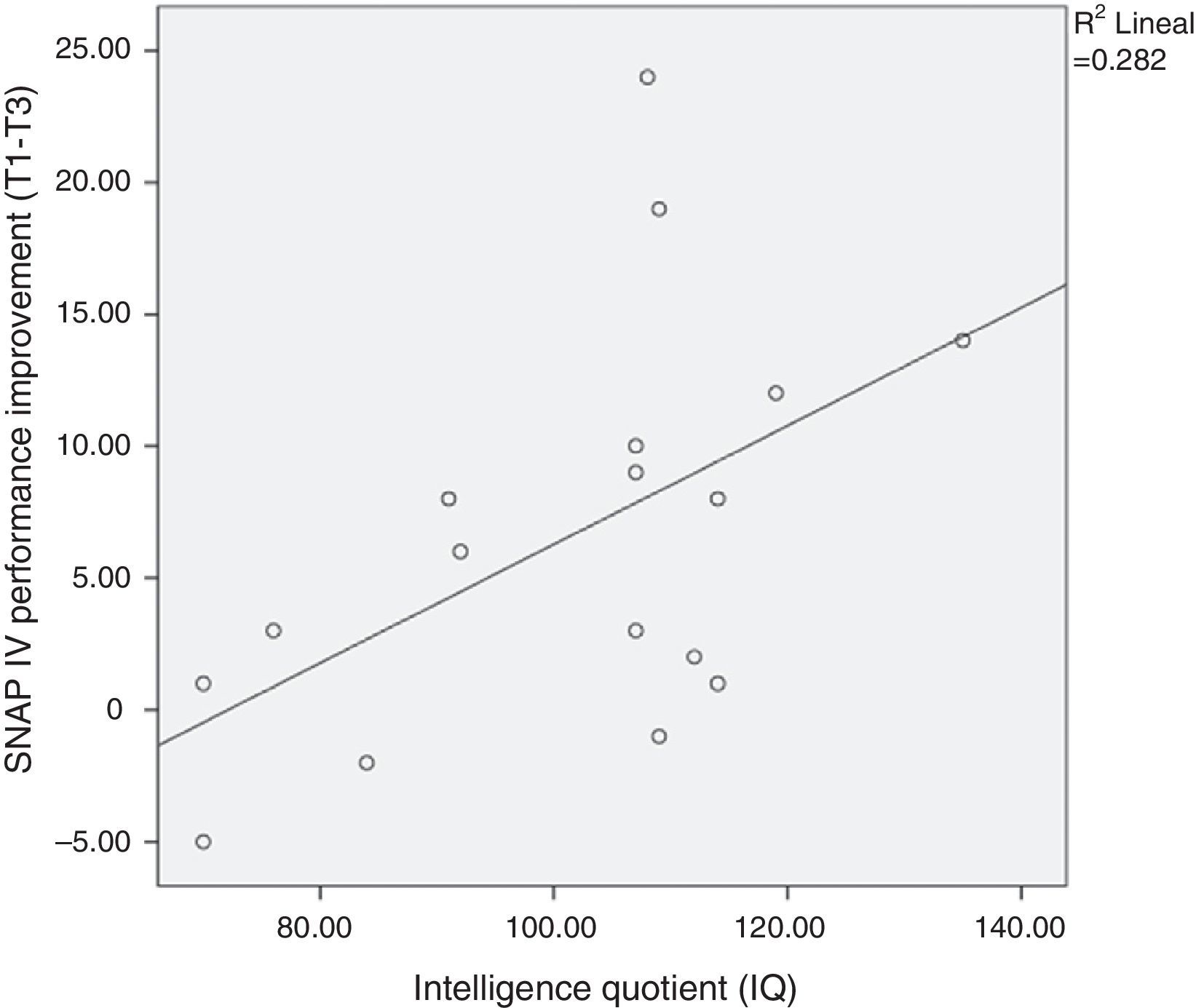
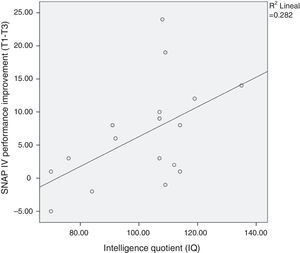
The improvement in ADHD severity scores from baseline did not vary by age, commitment (as measured by chess attendance), and hours of practice at home. However, as expected, we found a correlation between intelligence quotient (IQ) and SNAP-IV improvement (p<.05; see Fig. 2).
DiscussionThis pilot study showed statistically significant clinical improvements in both inattentive and hyperactive symptoms from pre- to post-chess training in 44 children and adolescents with ADHD. A significant majority of parents reported some improvement in ADHD severity in their children, and nearly half of the parents reported a 30% decrease in ADHD severity. In addition, effect size estimates in the present study were large, similar to the effect sizes of methylphenidate and psychosocial treatments reported in a recent meta-analysis,12 but lower than the effect size of lisdexamfetamine dimesylate for treating ADHD in both children and adults.31–33 Although the majority of children received benefits from chess training, our results suggest that the greater the intelligence of the child, the more important the improvement in ADHD symptoms.
Overall improvement in ADHD with chess trainingConsistent with our hypotheses, we found a decrease in the mean score versus the baseline for both parent-rated scales after chess training. Both inattention and hyperactivity/impulsivity symptoms improved according to the pre- and post-treatment results. Unfortunately, it is difficult to put into context our findings because there are no scientifically sound studies on the putative therapeutic effect of playing chess in children and adolescents with ADHD. However, a previous study has established that chess can help improve social and cognitive skills in children.34 A recent study suggests the beneficial effects of chess playing compared to other extracurricular activities in children. The authors compared 170 school children between the ages of 6 and 16 years who played chess as an extracurricular activity with 60 children playing either soccer or basketball after school. They reported that those playing chess were more likely to improve their cognitive abilities, problem-solving capacity, and coping in contrast to the sports group.35 Furthermore, chess improved object relationships in narcissistic adolescents.36 Also, in a study carried out in New Jersey, the authors reported that chess enhanced students’ self-esteem after just one year of playing chess.37 An alternative explanation for the observed reduction in SNAP/Conners scores could be a placebo effect (see strengths and limitations).
Given that we did not measure executive functions, we can only speculate if our results are a product of an improvement of executive functions in ADHD children. Executive functions include some cognitive processes core to ADHD (i.e. working memory, inhibition, multi-tasking, monitoring of actions).20,38,39 Interestingly, some executive functions are involved when playing chess (i.e. the ability to think ahead, analyze the effectiveness of moves made, and plan chess moves before they are made).21 Furthermore, the caudate nucleus, a structure involved in the fronto-striatal circuitry, is an area of the brain most frequently associated with ADHD40–42 that may cause impaired executive functions.43 Recently, it was reported that chess experts present structural changes in the caudate nucleus that might explain a greater behavioral control.44 Another reason that help to explain why chess is beneficial to ADHD children is that these children benefit from immediate feed-back and structure.45
GenderClinical improvement was found in both genders. This is important because we have recently reported that the female gender is a marker of a worse prognosis in children diagnosed with hyperactivity disorder (ICD-10).6 Hyperactivity is frequently a “hidden disorder” in girls. Even though more boys than girls are diagnosed with ADHD,46 both genders equally respond to psychostimulant treatment.47 As suggested here, girls can also benefit from chess training.
Pharmacologically treated or de novo ADHD childrenIt is also important to stress that the beneficial effects of playing chess were found in both pharmacologically treated and de novo ADHD children. Pharmacologically treated ADHD children displayed higher severity ratings in both scales than de novo ADHD children at T1. This is not surprising, as pharmacological treatment is the treatment of choice in children with severe ADHD.10 Our clinical impression is that, at least for the most severe ADHD children, medication is necessary to decrease the level of ADHD symptoms for children to benefit from chess training, at least initially. In other words, playing chess appear to have a similar role to psychotherapy within the multimodal treatment schema for children with ADHD.
Intelligence and improvementContrary to our expectations, we did not find a dose–response relationship between the number of hours of chess practiced at home and amelioration of ADHD symptoms. Thus, the improvement of ADHD with chess training in our study should be explained by other factors not recorded in our study–for instance, if children practiced against other people or the chess-machine–or intelligence. As expected, we found a correlation between IQ and ADHD improvement after the chess course. This is intuitively coherent with the fact that the more intelligent children may find it easier to implement the complex strategies necessary to win at chess. Furthermore, in a recent study, the authors reported strong evidence for their hypothesis that adults with high IQ who meet DSM-IV criteria for ADHD are more likely to report more impairment in executive functions than the general population.48 Therefore, playing chess could be a very interesting therapeutic complement to high IQ patients with ADHD
Advantages of chess training for ADHD treatmentCompared to other treatments for ADHD, chess training presents several advantages. First, it is cheaper than available psychotherapies. Second, playing chess has no side effects, and consequently children and parents easily accept it. Many children discontinue ADHD medication due to either psychological side effects or perceived inadequate effectiveness.49 Moreover, side effects can negatively impact student performance in school.50 Third, play is critical to the social development of children.51 These authors reported that the key factor for play-based interventions is their ability to capture the motivation of children with ADHD, and we concur. Indeed, motivation is critical for the success of any treatment.52 Not surprisingly, the majority of children entering in our study were highly motivated to play chess, as demonstrated by the low rate of chess discontinuation (less than 5%). This is particularly important, given that children diagnosed with ADHD have difficulties in social play, including lack of interpersonal empathy while playing.51
In any case, given that the level of inattention is heightened when tasks are boring,51 playing chess is not recommended for those ADHD children that spontaneously express that they do not like playing chess. Children who lack motivation for a particular task (i.e. chess playing) will probably be unable to sustain attention on this task.53
Strengths and limitationsTo the best of our knowledge, this is the first study supporting the hypothesis that playing chess can improve ADHD symptoms. Another strength is that the pre and post-intervention design allows for the comparison of ADHD severity. Even using the 30% ADHD improvement criteria,54 a significant percentage of parents reported that the ADHD severity decreased in both scales. This is important considering that reductions between 20% and 25% in the severity of symptoms are considered clinically meaningful.55,56 Moreover, we used effect sizes to measure the magnitude of the association between chess training and the improvement in ADHD symptoms, which enables to measure this type of relationships more effectively than p values, regardless of statistical significance.57 But effect sizes “satisfy statistical, not clinical, needs”.58 Indeed, even if our results are promising, we should bear in mind that the level of post-training ADHD symptoms displayed by most of our patients was still notable.
These results must also be interpreted in light of several limitations inherent to naturalistic, descriptive studies.59 First, the outcome measures were two parent-reported scales. Parents’ expectations of the intervention's outcome may bias their responses.60,61 However, all parents were blind to their previous assessments, and some have suggested that the expectation of treatment benefit does not contribute to changes in treatment response in ADHD children.62 Given that resources are very limited in our media, we considered that the descriptive intervention design was the most feasible at this point in time. In addition, randomized, controlled trials also have limitations.59 Second, we did not use clinical interviews such as the Diagnostic Interview Schedule for Children, version IV (DISC-IV) to corroborate our clinical diagnosis. However, ADHD is a clinical diagnosis,63 and both the SNAP-IV and the CPRS-HI are ecologically valid dimensions of the child's behavior at home.64 Finally, the sample size was relatively small. Although, we feel that the sample size was fair enough given the pilot nature of the present study. In conclusion, more methodologically sound studies (i.e. including independent observations; randomized, controlled trial design studies) with larger samples are warranted.
ConclusionsThis pilot study presents preliminary evidence suggesting that chess playing might play a role in the treatment for children and adolescents with ADHD. High IQ children appear to be particularly benefited. Given the weaknesses of our study–open label, no control group, no randomization, and small sample size–our results should be interpreted with caution. This pilot project highlights the importance of carrying out larger studies with a case–control design. If our results are replicated in more robust studies, playing chess could be included within the multimodal treatment of ADHD. Alternative therapies such as chess training might be particularly interesting in developing or impoverished countries where resources are scarce.8 Thus, “chess therapy” for ADHD could be potentially used as a low cost resource for struggling ADHD students in the public school system.
Ethical responsibilitiesProtection of human and animal subjectsThe authors declare that procedures conformed to the ethical standards of the responsible committee on human experimentation and in accordance with the World Medical Association and the Helsinki Declaration.
Confidentiality of dataThe authors declare that they have followed the protocols of the workplace on the publication of data from patients.
Right to privacy and informed consentThe authors have obtained the informed consent of patients and/or subjects referred to in article consent. This document is in the possession of the corresponding author.
Conflict of interestIn the last three years, Dr. Hilario Blasco-Fontecilla has received lecture fees from Eli Lilly.
AB-Biotics, Janssen, Rovi, and Shire. The remaining authors report no conflict of interest.
Please cite this article as: Blasco-Fontecilla H, Gonzalez-Perez M, Garcia-Lopez R, Poza-Cano B, Perez-Moreno MR, de Leon-Martinez V, et al. Eficacia del ajedrez en el tratamiento del trastorno por déficit de atención e hiperactividad: un estudio prospectivo abierto. Rev Psiquiatr Salud Ment (Barc). 2016;9:13–21.