Bacterial superinfection, as well as ventilation associated pneumonia (VAP), are both frequent events in critical care. During the COVID-19 pandemic, usual diagnostic practices such as bronchoalveolar lavage and tracheal aspirate are limited due to their associated high risk of exposure for the operator. In order to set primary focus on the protection of health care personnel, a modified tracheal aspiration (M-TA) technique was developed and used for acquiring microbiological samples from the lower respiratory tract using a closed suction device.
MethodsRetrospective observational study was conducted to evaluate effectiveness of an M-TA.
ResultsA total of 33 M-TA samples were analysed. In 66.6% of the cases, results led to a change in medical decision making. A 100% accuracy was achieved regarding COVID-19 diagnosis, and a 56% bacterial growth-rate in cultures where VAP was suspected. No health care personnel developed symptoms or tested positive for COVID-19 during or after sample collection.
ConclusionThe M-TA technique presented could be considered as a safe and effective procedure with low percentage of complications.
Tanto la sobreinfección bacteriana como la neumonía asociada a la ventilación (NAV) son eventos frecuentes en los cuidados críticos. Durante la pandemia de COVID-19, las prácticas diagnósticas habituales, como el lavado broncoalveolar y el aspirado traqueal, están limitadas debido al alto riesgo de exposición que conllevan para el operador. Con el fin de poner el foco principal en la protección del personal sanitario, se desarrolla y utiliza una técnica de aspiración traqueal modificada (M-TA) para la adquisición de muestras microbiológicas del tracto respiratorio inferior con un dispositivo de aspiración cerrado.
MétodosSe realiza un estudio observacional retrospectivo para evaluar la eficacia de la M-TA.
ResultadosSe analizaron un total de 33 muestras de M-TA. En el 66,6% de los casos, los resultados condujeron a un cambio en la toma de decisiones médicas. Se alcanzó una precisión del 100% en el diagnóstico de COVID-19 y una tasa de crecimiento bacteriano del 56% en las cultivas en las que se sospechó de NAV. Ningún personal sanitario desarrolló síntomas ni dio positivo a COVID-19 durante o después de la recogida de muestras.
ConclusiónLa técnica de M-TA presentada podría considerarse como un procedimiento seguro y eficaz con bajo porcentaje de complicaciones.
Coronavirus Disease 2019 (COVID-19) was defined as pandemic by the World Health Organization1 and affected more than 20 million people globally with confirmed cases in 215 countries. Since the beginning of the outbreak, the risk of viral transmission to healthcare personnel at the front line has been a global concern2 posing a challenge for healthcare systems in terms of managing human resources, supplies, and personal protective equipment. Therefore, it is key that all clinical decisions are based on prevention strategies to obtain the best value from the available resources.3
The collection of samples from the surface of the respiratory mucosa with nasopharyngeal swabs is a standard procedure used for diagnosis of COVID-19 in adults and children. Nevertheless, early data suggested relatively poor sensitivity of initial reverse transcription polymerase chain reaction (RT-PCR) tests from swabs.4 False-negative results of nasopharyngeal swabs have direct implications for infection control and isolation rooms management. In addition to the previous, among critically ill patients the bacterial superinfection as well as a ventilator-associated pneumonia (VAP) are frequent events,5 and usual practices such as bronchoalveolar lavage and traditional tracheal aspiration are limited due to their associated high risk of exposure for the operator.6,7
Setting primary focus on the protection of health care personnel, a modified tracheal aspiration (M-TA) technique is developed and used for acquiring a lower respiratory tract microbiological sample with a closed suction device. This technique proved to be useful not only in aiding COVID-19 diagnostic confirmation in patients with negative RT-PCR from swabs, but also in other respiratory infectious diseases when COVID-19 status remains unknown.
MethodsA retrospective analysis was conducted in medical records of patients with suspected or confirmed COVID-19 admitted to the intensive care unit (ICU) of high complexity between June 1, 2020 and August 1, 2020. All patients included in the analysis were more than 18 years-old, underwent mechanical ventilation (MV), and required a lower respiratory tract microbiological sample due to suspected VAP or COVID-19 diagnosis. A description of epidemiological data, prior nasopharyngeal swab test-results and microbial rescue of M-TA from patients’ medical records was included.
The data collection for this study was completed as part of the ICU follow-up clinic up to August 1st, 2020. This study was approved by the Ethics Committee of Hospital Italiano de Buenos Aires in June 2020, under protocol number 5678.
Study definitionsIn patients with confirmed COVID-19 in whom VAP was suspected, an M-TA was performed with the aim of microbiologically defining VAP. Otherwise, in patients with clinical suspicion of COVID-19 and an initial negative nasopharyngeal swab test, a M-TA was performed with the aim of achieving COVID-19 diagnosis. Also, in this group of patients, the true false reports were confirmed with serologic detection of IgG and IgM against SARS-CoV-2.
VAP suspicion was determined by the treating physician and was based on: new and persistent (48-h) or progressive radiographic infiltrate plus two of the following: fever (temperature≥38°C), blood leukocyte count≥10,000cells/ml, purulent tracheal secretions, and gas exchange degradation with increasing levels of positive end-expiratory pressure (PEEP) or fraction of inspired oxygen.8 VAP was microbiologically defined, when the M-TA collected reached a count ≥105 colony forming units per millilitre (UFC/ml). Only samples with less than 10 epithelial cells per field (100x magnification) were considered.
Suspected COVID-19 was determined following the Argentine National Ministry of Health definition.9 COVID-19 diagnosis was microbiologically confirmed when RT-PCR from nasopharyngeal swabs or M-TA tested positive for SARS-CoV-2. All laboratory tests were processed in a College of American Pathologist-accredited laboratory.
Changes in medical decisions such as indication or withdrawal of isolation, initiation or suspension of antibiotics, and modifications in the antibiotic therapy were analyzed. Complications associated with the procedure were documented.
All healthcare personnel involved in performing M-TA underwent serologic testing for COVID-19 every 2 weeks during the study period.
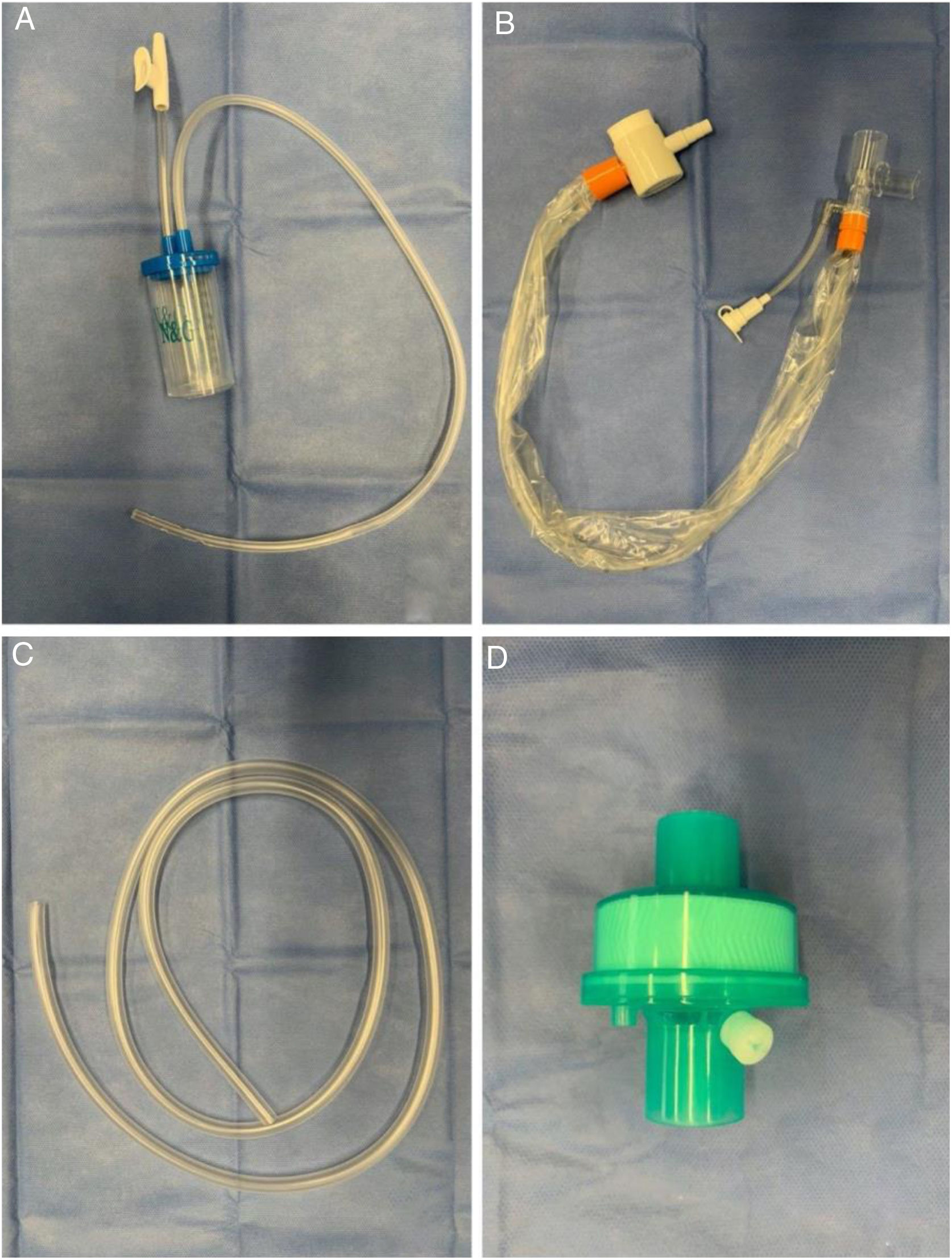
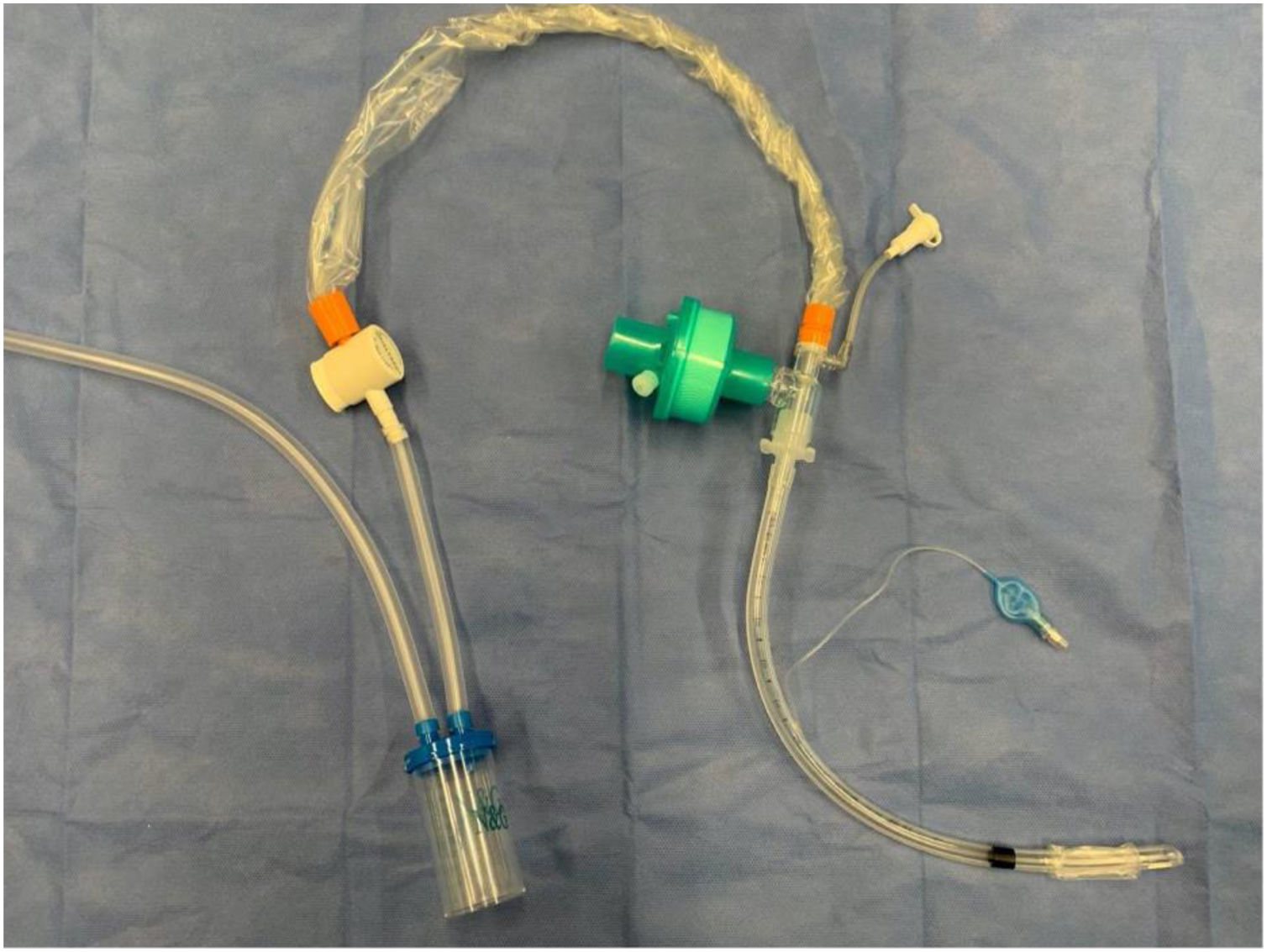
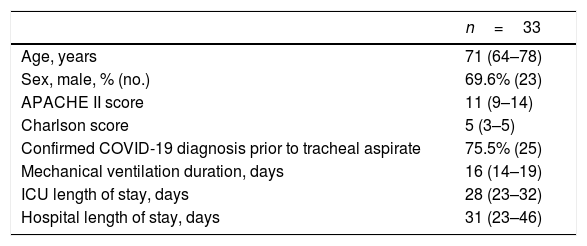
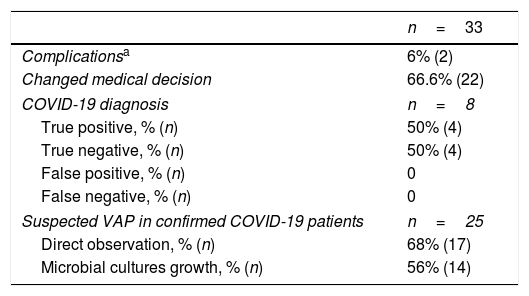
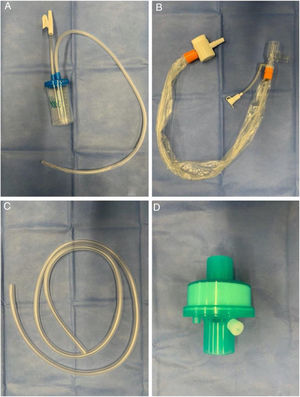
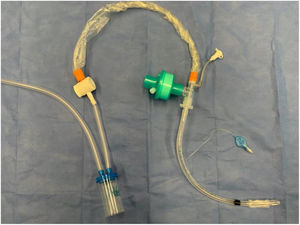
Modified tracheal aspiration techniqueIn order to perform tracheal aspiration, 2 operators donned with personal protective equipment (gown, cap, goggles, gloves, N-95 mask or equivalent, visor and second pair of gloves were required) entered the patient's room where a high-efficiency particulate air filter was used. The collection of M-TA was performed using a 14 French siliconized polyvinyl-chloride (PVC) probe with a closed endotracheal suction system (Halyard Health®, United States) assembled with a sterile polypropylene collector bottle (CEEMED®, Argentina). A heat & moisture exchanger filter (HMEF) (Besmed®, Taiwan) was used to avoid virus particles dispersion, and a sterile T-63 tubing (BIOM®, India) was used for connecting pieces (Figs. 1 and 2). The PVC probe was introduced through the endotracheal tube or tracheostomy cannula until resistance was encountered (level of the carina in the trachea). This was followed by the release of the vacuum, and the probe was delicately removed using turning movements, until secretions were aspirated into the collector bottle. No saline solution was used for liquefy secretions, and strictly aseptic principles were followed.
The following were the steps the suggested to perform tracheal aspiration:
- 1.
Explain the process to the patient or relatives.
- 2.
Check all materials are prepared before entering the patient's room (collection bottle, siliconized polyvinyl-chloride probe with a closed endotracheal suction system, T63 tubing, heat & moisture exchanger filter [HMEF]).
- 3.
Make a closed aspiration device as follows, maintaining sterile management at all times and taking care for the main operator to remain in sterile conditions throughout the procedure:
- 3.1
Cut the tubings of the collection bottle and discard them.
- 3.2
Cut T63 tubing into two pieces: a short one of about 10cm, and a long one of at least 1m.
- 3.3
Connect one extreme of the short piece of T63 tubing to the collection bottle.
- 3.4
Connect the other extreme of short piece T63 tubing to the closed endotracheal suction system.
- 3.5
Connect HMEF to the tracheal suction system.
- 3.1
- 4.
Check that the closed endotracheal suction system security valve is closed.
- 5.
Check hemodynamics and pre-oxygenate with FiO2 1.0.
- 6.
Initiate deep sedation and analgesic drugs. Neuromuscular blockade should be required for suppressing cough reflex.
- 7.
Check absence of ventilatory drive in CPAP mode.
- 8.
(Second operator) Set the ventilator in “stand-by” to avoid the main operator losing sterility.
- 9.
(Second operator) Clamp the orotracheal tube with a Kocher clamp. If the patient has a tracheostomy, this step should be omitted.
- 10.
(Second operator) disconnect the ventilator tubing from the orotracheal tube/tracheostomy cannula.
- 11.
Connect the closed aspiration device to the orotracheal tube/tracheostomy cannula.
- 12.
Connect ventilator tubing to the closed aspiration device.
- 13.
(Second operator) Connect the loose extreme of the long piece of T63 tubing to the room vacuum system.
- 14.
Open the tracheal suction system security valve.
- 15.
(Second operator) Unclamp the Kocher clamp.
- 16.
(Second operator) Restart ventilation.
- 17.
Take a respiratory sample by aspirating secretions.
- 18.
Close the tracheal suction system security valve.
- 19.
Disconnect the tracheal aspiration collection bottle and the short T63 piece from the closed aspiration device.
- 20.
Set the alarms, secure adequate FiO2 and verify that the humidification system is working.
- 21.
Labeled the microbiological sample with patient's data as well as with the legend “COVID-19” for its proper processing.
- 22.
Dof personal protective equipment.
- 23.
Perform hand washing.
Continuous variables were expressed as medians and interquartile ranges or simple ranges, as appropriate. Categorical variables were summarized as counts and percentages. No imputation was made for missing data. Given the fact that the cohort of patients in the study was not derived from random selection, all statistics are deemed to be descriptive only. RStudio developed by R-Tools Technology Inc was used for analysis.
Funding sourceStudy did not receive funding sources.
Results33 patients were included in the study; 10 were female (30.3%) and 23 men (69.6%). Median age was 71 years (interquartile range 64 - 78). Among the overall population, in 75.5% (25) of the cases, M-TA was performed due to VAP suspicion, and in 24.4% (8) of the cases M-TA was performed for COVID-19 diagnosis. Demographic and clinical characteristics of patients are shown in Table 1.
Patient characteristics.
| n=33 | |
|---|---|
| Age, years | 71 (64–78) |
| Sex, male, % (no.) | 69.6% (23) |
| APACHE II score | 11 (9–14) |
| Charlson score | 5 (3–5) |
| Confirmed COVID-19 diagnosis prior to tracheal aspirate | 75.5% (25) |
| Mechanical ventilation duration, days | 16 (14–19) |
| ICU length of stay, days | 28 (23–32) |
| Hospital length of stay, days | 31 (23–46) |
Continuous variables are presented as median (and interquartile range), and categorical variables as count (%).
APACHE II, Acute Physiology And Chronic Health Evaluation II.
Of the 33 samples obtained, 22 (66.6%) led to a change in medical decision making. When technique was used for COVID-19 diagnosis, among a total of 8 patients 4 (50%) were a true positive and 4 (50%) true negative. Analyzing microbiological samples due to suspected VAP in confirmed COVID-19 patients, 14 out of 25 samples (56%) presented microbial growth in cultives. Among all 33 procedures performed, only 2 associated complications (6%) were observed: one hypotension episode due to propofol bolus, and one inability to obtain the sample due to absence of secretions. Results of M-TA are shown in Table 2.
Results.
| n=33 | |
|---|---|
| Complicationsa | 6% (2) |
| Changed medical decision | 66.6% (22) |
| COVID-19 diagnosis | n=8 |
| True positive, % (n) | 50% (4) |
| True negative, % (n) | 50% (4) |
| False positive, % (n) | 0 |
| False negative, % (n) | 0 |
| Suspected VAP in confirmed COVID-19 patients | n=25 |
| Direct observation, % (n) | 68% (17) |
| Microbial cultures growth, % (n) | 56% (14) |
Finally, none of the four healthcare workers (0%) involved in performing M-TA developed symptoms nor tested positive for COVID-19.
DiscussionIncidence of negative RT-PCR results in SARS-CoV-2-positive patients is likely under-reported. Due to the high risk of viral spread, an initial negative nasopharyngeal swab test should not alter clinical management in patients showing the constellation of symptoms consistent with COVID-19. The M-TA technique developed, achieved a 100% effectiveness rate regarding COVID-19 diagnosis. The true false reports were confirmed with SARS-CoV-2 serologic studies so it is advised that when feasible, lower respiratory tract samples should be collected in order to help confirm the diagnosis.10
In the event that VAP was suspected, there was a high rate of germ rescue in the samples obtained. A higher revenue rate was observed in comparison with the one reported for bronchoalveolar lavage and conventional tracheal aspirate technique.12 It is relevant to pinpoint that a key factor in decreasing mortality associated with VAP is the administration of adequate antibiotics as early as possible.11
Even though the size of the sample is small, the procedure described could be considered safe and effective with a low percentage of associated complications. Moreover, the technique developed involves minimal additional costs because it requires materials that are widely available.
Finally, it is worth to mention that none of the healthcare personnel involved have developed symptoms nor tested positive for COVID-19 during or after the data collection.
ConclusionM-TA technique presented could be considered a safe and effective procedure with low percentage of complications.
Authors’ contributionSofía Schverdfinger, Indalecio Carboni Bisso: Conceptualization, Methodology, Formal analysis, Investigation, Writing – Original Draft.
Romina Famiglietti, Marcelo Di Grazia: Data Curation.
Sabrina Di Stefano: Writing – Review & Editing, Supervision.
Marcos José Las Heras: Project administration.
FundingAuthors received no specific funding for this work.
Conflict of interestsAuthors have declared that no competing interests exist.
The research team wants to thank Ignacio Fernández Ceballos, Pablo Beber, Maria de los Ángeles Magaz, Iván Huespe, Carolina Lockhart, Agustín Massó, Julieta González Anaya, Micaela Hornos, Marcela Ducrey, Pablo Coria, Germán Mayer and Juan Martín Nuñez Silveira for their valuable collaboration in this work.