INTRODUCTION
During the last few years numerous works have been published, such as those included in a recent review1 indicating an interest in finding new treatment schedules for the administration of subcutaneous immunotherapy. They all had the objective of trying to reach a maintenance dose in the shortest time with the least number of doses possible, and without posing an increased risk of adverse reactions in the patient. Also, the search for these new schemes has the other important objective of reducing indirect costs as consequence of administration of allergen vaccines, as much for the patient as for the Immunotherapy Unit, or Allergy Service where this type of treatment is administered.
These new schedules, depending on the time necessary to reach the maintenance dose, and on the interval between each dose group, are grouped under different headings: rush, ultra-rush and cluster. The first two have been used fundamentally for the treatment of allergy to Hymenoptera venom, while cluster schedules have been for these venoms as well as different pneumoallergens: mites, pollens, epithelia and moulds2-7.
A common characteristic to the aforementioned therapeutic schemes, is that their administration should be done under the direct supervision of the specialist. However, it is known that in many cases, depending on such factors as the severity of the allergic disease, the degree of patient's sensitisation, the extract type etc., the subcutaneous administration of allergenic vaccines is carried out in health centres, outside the direct control of an allergy specialist. For this reason, it is of great interest to find a treatment schedule that, following the norms of administration by a conventional rule (1 dose per week), will be able to reduce significantly the number of doses in the initial phase without posing a greater risk of adverse reactions for the patient.
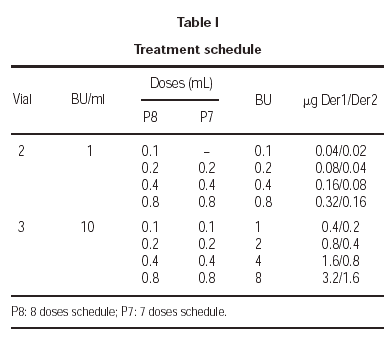
In the last few years, the ALK-ABELLO group, with the collaboration of a large number of clinical groups, has carried out different multicentred studies8-10, in which a cluster schedule has been developed that allows reaching the maintenance dose in 3 weeks (4 visits). Parallel to this development, the present multicenter study was carried out, in which a model of the conventional scheme was studied and starting with doses similar to the cluster schedule, and reached the maximum after the administration of 7 doses, instead of the 13 doses of the currently recommended conventional scheme.
MATERIALS AND METHODS
Patients
A total of 261 patients were included in 14 clinical groups. The inclusion criteria were: clinical history of perennial allergic rhinitis and/or asthma by sensitisation to mites (Dermatophagoides pteronyssinus and/or farinae) of at least 1 year duration, with positive cutaneous tests and/or serum specific IgE (≥ class 2, CAP Pharmacia, Uppsala, Sweden). Excluded from the study were those patients who had received previous immunotherapy, were sensitized to other clinically relevant allergens, who could not have the treatment administered under the supervision of an allergist, or where the administration of immunotherapy was contraindicated according to WHO criteria11.
Immunotherapy
The immunotherapy was administered subcutaneously using an extract of Dermatophagoides pteronyssinus and/or farinae (Pangramin Depot-UM, ALK-ABELLÓ, S.A., Madrid, Spain). In both cases, allergens extracts were adsorbed on aluminium hydroxide gel biologically standardized and with their major allergens (Der 1 and 2) quantified in Mass Units, using methods described by the manufacturer12.
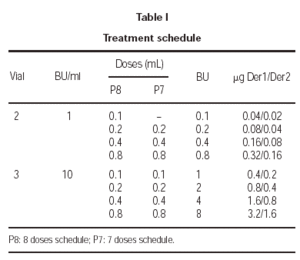
The treatment schedule during the increment dose phase was carried out as follows: the first patients, which included one from each of the participant groups, followed an 8 dose schedule and, once the tolerance of this rule was tested, the treatment for the rest of the patients was administered using a 7 dose schedule. Both schedules are shown in table I.
All doses were administered under the supervision of a specialist in each of the participant centres. The clinical situation of each patient was evaluated before administering each dose, according to the instructions contained in the EAACI Position Paper13.
In no case was premedication with antihistamines used before administering the corresponding dose.
Safety monitoring
The registration of adverse reactions was carried out according to the classification contained in the EAACI Position Paper13.
Statistical analysis
All the statistical analyses were carried out using the SAS system version 8.1. The association between the different variables was carried using the exact test of Fisher, having calculated the 95 % confidence intervals by means of the exact binomial method.
RESULTS
Sample characteristics
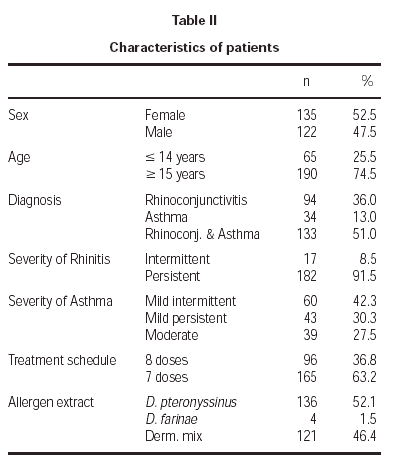
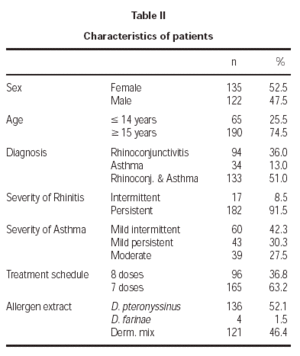
The patient characteristics are shown in table II. The mean age of the patients was 21.4 ± 9.3 years, and the average duration of allergic disease was 6.6 ± 5.4 years. A total of 10 withdrawals took place during the study, 3.8 % of the sample, of which only two occurred for poor tolerance. The remainder withdrew for various reasons, such as moving home, work problems, holidays, exacerbation of their asthmatic illness, and in 3 cases the cause was unknown.
Treatment characteristics
A total of 2290 doses were administered, of which 2015 corresponded to the initiation phase and 275 to the maintenance phase. For each of the two rules used, the number of administered doses was 931 for the 8 dose and 1359 for the 7 dose rule.
Tolerance
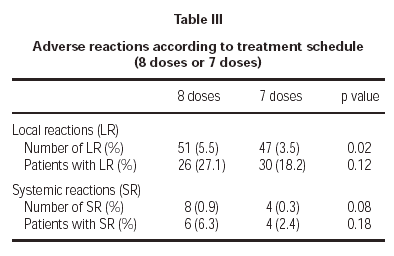
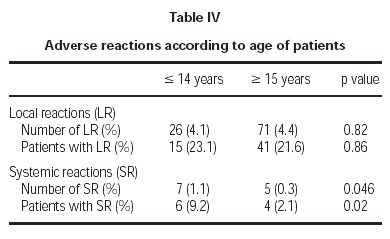
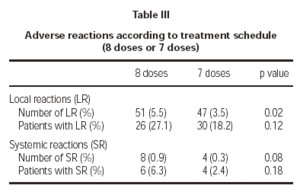
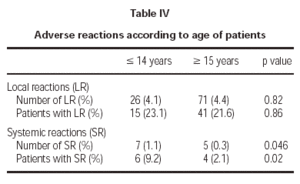
Of the total reactions recorded, 98 (89 %) were local, representing 4.3 % of the total doses administered. These reactions occurred in 56 patients (21.5 % of the sample). The remaining 12 reactions were systemic and were recorded in 10 patients, representing 0.5 % of the total number of doses administered and 3.8 % of the total number of patients. If we analyse the tolerance of the two schemes used, as shown in table III, a better tolerance is observed in the 7 dose schedule than that of the 8 dose. As for the age of the patients, it is shown in table IV that there are significantly more systemic reactions in patients less than 14 years of age than in those over this age.
The description of the systemic reactions is as follows: 5 asthmatic crises, 1 rhinitis, 1 angio-oedema (accompanied by rhinitis, cough and migraine), in two cases the reaction was described as wheezes and in 3 occasions there were unspecific symptoms. As for the time of appearance and the severity of the systemic reactions, according to the classification of the EAACI13 50 % occurred during the first 30 minutes after the administration of the immunotherapy and the remaining 50 % were delayed. As for the severity of systemic reactions, of the immediate ones, all were grade 2 except one (angioedema) which was classified as grade 3. As for the delayed ones, all were registered as mild or moderate.
Of the 12 reactions, 10 responded perfectly to rescue medication, and were able to continue with the administration of the immunotherapy. In one case it was necessary to give a lower maintenance dose, and in another, who presented with two asthmatic crises in two consecutive doses, it was decided to suspend treatment.
All the systemic reactions took place in patients with asthma: 5 in patients with the exclusive diagnosis of asthma and 7 in patients diagnosed with rhinitis and asthma.
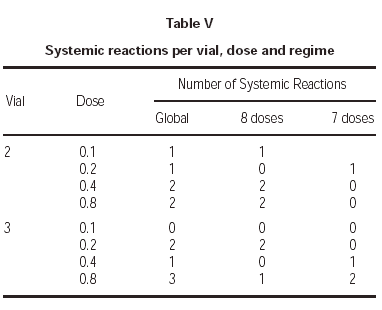
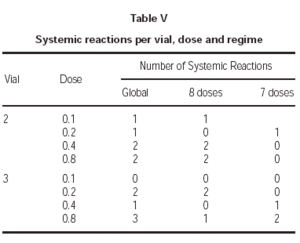
Lastly, one of the most relevant aspects is to know in which treatment phase, dose and vial were the systemic reactions registered. As for the first point, all except one occurred during the increment dose phase. As regards the vial, there were no significant differences, as 6 occurred during the administration of the vial 2 and 6 with the vial 3 having the maximum concentration. With respect to the doses in which the reactions occurred, the distribution found can be seen in table V.
DISCUSSION
The first objective of the study was to check the tolerance of the proposed rules. If we compare the results with those published in other articles using an extract identical to the one which was administered here, but injecting it under the conventional 13 dose schedule, we observe that Tabar et al14 in 226 patients in which 5120 doses were administered, encountered systemic reactions in 0.5 % of the doses, identical to that found in the present study. Also González de la Cuesta et al15 with 88 patients and 1244 administered doses obtained 0.32 %. In another study carried out using an extract of Dermatophagoides pteronyssinus and/or farinae measured in Mass Units, (data which has not yet been published), with 200 patients and 1902 doses administered under a cluster rule, the number of systemic reactions by dose was 0.3 %. Therefore, we can see that the tolerance of the new proposed outline is similar to the one obtained with other administration rules.
A second point was to find out which of the two proposed schemes (8 and 7 dose) presented with a better tolerance profile. The 8 dose rule, as compared to the 7 dose one, registered a significantly greater number of local reactions and less significantly, a larger number of systemic reactions. Therefore, it seems that the 7 dose rule has been tolerated better by patients.
A third important fact is the greater frequency of adverse reactions registered in the child population. Although the previously detailed systemic reactions were not serious and the only withdrawal that took place was an adult, they appeared with more frequency in patients younger than 14 years than in those older. However, given the casuistry difference between patient samples, and as the study was not designed with the objective of differentiating between both age groups, a more specific study would be necessary to evaluate this point appropriately.
The last point to highlight is the saving that this new rule represents with regards to the conventional rule of 13 doses. If the patients in the present study had immunotherapy administered under the 13 dose scheme and without considering the need to repeat doses due to adverse reactions or other causes, a total of 3393 visits would have been necessary to reach the maintenance dose. With the 7 dose schedule, which we consider is more suitable for the administration of the extract, 1827 visits would be necessary, representing a 44 % decrease in indirect costs incurred by using this type of treatment.
Therefore, in view of the results found, we conclude that the administration of mite extract quantified in Mass Units using a conventional 7 dose scheme presents with an excellent tolerance profile, similar to that of the conventional 13 dose scheme, and represents a substantial decrease in the number of visits necessary to reach the maintenance dose.