INTRODUCTION
Formoterol fumarate is a new, long-acting inhalatory β2-agonists that cause bronchodilation for 8 to 12 hours. Whereas albuterol is a β2-agonist with a rapid onset and a duration of action for approximately 6 hours after a single dose1,2. In order to know whether these differences may result clinically relevant in the emergency room, we compared the effect of formoterol and albuterol, both administered with a Turbuhaler® system in paediatric patients..
MATERIALS AND METHODS
The Research and Ethics Committees at the Hospital Infantil de México "Federico Gómez" approved the study. The study protocol was explained after patient was controlled, and written informed consent was obtained from all parents or guardians. The study was performed according with the principles stated in the Declaration of Helsinki according with updated version of Edinburgh 2000 of the Declaration of Helsinki of the World Medical Association.
Inclusion criteria met for participating children were age between 5 to 15 years old, with a confirmed diagnosis of asthma as defined by the American Thoracic Society6. Exclusion criteria were patients on oral bronchodilators, long-acting inhaled β2-agonist, or oral glucocorticoids 12 hours before the test, or with respiratory infections.
It was required that the patients had mild acute asthma at the moment of the test as well as presented normal conscious state, respiratory rate > 30 % than its basal value, oxygen saturation (SaO2) < 94 %, without paradox pulse nor using accessory thoracic muscles. The baseline FEV1 had to be at least 70 % of the predicted values.
By means of a pre-designed table of random numbers, 38 patients were located in one of the two study groups to receive either formoterol 12 μg or albuterol 200 μg (Astra México SA de CV), both supplied in identical canisters and administered via a Turbuhaler system®
A spirometry was performed according with the American Thoracic Society criteria7, with a spirometer (Spirotech model S-500, Inc.) with a pneumotachograph head and pressure transducer attached to an on-line computer. Determination of the forced expiratory volume in one second (FEV1) was registered. The peak expiratory flow (PEF) was also obtained. The children performed three forced expiratory maneuvers from total lung capacity to residual volume, and the best test value was used for statistical analysis. The FEV1 was measured before, 3, 30 and 60 minutes after drug administration.
Information about adverse events was obtained from patients by means of a self-reported written questionnaire answered by parents and, when possible, by the patient.
Data analysis
Data were summarized as mean ± SD. Differences between treatments were investigated by means of a two factorial ANOVA. Confidence intervals (95 % CI) of the differences were calculated by standard procedures8.
RESULTS
Formoterol was administered to 18 children (11 males, 9 females) aged 8.9 ± 3.4 years (range 5 to 15 years), weight 32.5 ± 14.2 kg (16 to 67 kg) and height 138.8 ± 15.2 cm (110 to 163 cm). Albuterol was administered also to 18 children (11 males, 9 female) aged 8.7 ± 2.3 years (5 to 15 years), weight 37.7 ± 13.8 kg (21 to 72 kg) and height 134.2 ± 15.7 cm (110 to 169 cm).
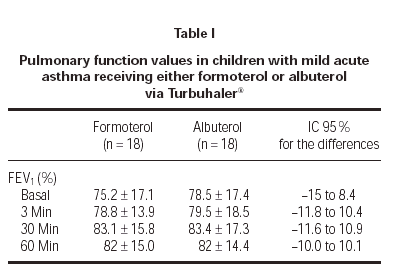
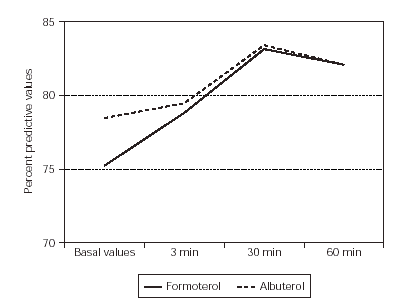
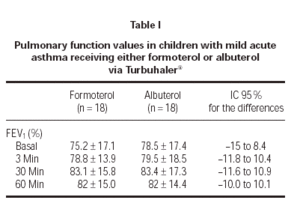
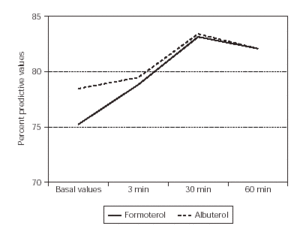
The %FEV1 basal values at 3, 30 and 60 minutes values were similar (p = 0.65) between formoterol and albuterol (table I and figure 1). There were no adverse events of clinical relevance or causally related to treatment.
Figure 1.--The means of FEV1 values of two groups patients.
DISCUSSION
The results demonstrated that 12 μg formoterol and 200 μg albuterol have similar bronchodilatory effect in pediatric patients with mild acute asthma when administered via Turbuhaler system®. Inhalation is the most direct route for drug delivery in asthma therapy and leads to a rapid response of bronchodilators9. Dose-inhaler are the most commonly used devices, but dry-power inhalers such as Turbuhaler® are being used more frequently. Since the dry-power inhalers were introduced, several studies have documented the efficacy of their use with short-acting bronchodilators in10 children. The current data thus shows that formoterol via Turbuhaler system® has a rapid onset of action, comparable with the short-action β2-agonist albuterol, at doses used herein.
ACKNOWLEDGMENTS
The authors are grateful with Astra México SA de CV for supplying the formoterol and albuterol.