INTRODUCTION
Allergen immunotherapy (AIT) is still a controversial point for some groups1,2, in spite of many researches that guarantee its clinical effectiveness in case of respiratory allergy due to of pollens3-5, mites6,7, moulds8,9, animal dander10,11, and in hymenoptera anaphylaxis12,13. The consensus documents14,15, or metaanalysis16 locate it already in an evidence- based medicine level17.
However, and this is one of the arguments that can be used against, the AIT, as any other therapeutic form, it is not risk free and serious systemic reactions can occur during its administration. The majority of studies about safety with the AIT are retrospective1,18 and they use different methods, frequently far from clinical daily practice19,20. That is the reason why we decided to carry out a prospective study that let us know and reduce risk factors concerning adverse reactions, especially systemic, produced during the routine procedure of AIT administration in our Immunotherapy Unit. In this unit we work with a very well trained nursery staff (more than 3000 doses administered per year), we use only standardized extracts and we dispose of an appropriate training or allergists, immunotherapy clinic support staff and a computerized data recollection21, as well as necessary material to treat an eventual anaphylactic reaction, according to the European Academy of Allergology and Clinical Immunology (EAACI)14 recommendations.
METHODS
Patients
We evaluated 273 consecutive patients between 4 and 65 years of age, both included (an average of 23.42 ± 15.79 years old), with indication of immunotherapy: 264 for respiratory pathology and 9 for hymenoptera anaphylaxis. 28 of the patients (10 % of the sum) with respiratory allergy exclusively showed rhinoconjunctivitis and the rest were asthmatic (85.2 %), 33.3 % of these with intermittent asthma, 43.7 % with persistent mild asthma and 22.9 % with persistent moderate asthma according to NHLBI/WHO consensus22. Patients maintain the necessary symptomatic treatment for their clinical stability during the course of the IT. The following patients' variables were analysed: age, sex, diagnostic (and severity in asthmatic), sensitizing allergens, PD20 methacholine and environmental conditions (origin of the population, presence of pets at home, kind of house and level of antigen exposition).
Immunotherapy
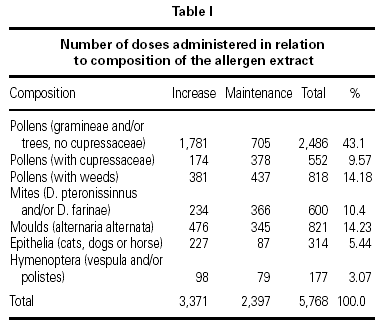
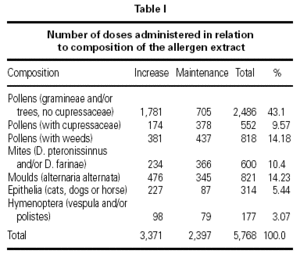
The vaccines used were all standardized in accordance to the International Consensus23,24 and following conventional schedules. In every patient we tried to reach the maximum dose recommended by different manufacturers. The vaccines with modified extracts or allergoids were administered in a preseasonal regimen (from September to March), being all the rest administered following a continuous schedule. Of the total of administered vaccines 74.4 % were of pollens, 7.8 % of mites, 11 % of moulds (Alternaria alternata), 3.8 % animal dander (cat, dog or horse) and 3.2 % hymenoptera venom (Vespula or Polistes).
Doses Safety monitoring
Every dose was administered in our AIT unit following the recommendations of the AIT committee of the Sociedad Española de Alergología e Inmunología Clínica25 and the AIT subcommittee of the EAACI supervised by an allergologist. All data concerning each dose and the incidences were registered, always including the date-time, administered dose, PEF predose and 30 minutes later, symptoms 30 minutes later and local or systemic reactions in the previous dose, by the administrator nurse in a computing data base. Patients were asked before each dose about any reason that could indicate a modification in the schedule, including the illness activity, changes in medical treatment or appearance of a concomitant disease. We consider immediate local reactions those bigger than 5 centimetres in the initial minutes, and late systemic reactions those bigger than 10 centimetres. The gradations of immediate local and late reactions and the modification of the therapeutic regimen followed the EAACI recommendations.
Statistic
The collected data were processed in a data base (Access® data base Microsoft©). SPSS (Superior Performing Software Systems 10.0) was used for statistical analysis. Comparative analysis of groups was carried out by the chi-square nonparametric test and the exact Fisher's test. For the multivariable research we followed every step of the logistic method. A P value of < 0.05 was considered statistically significant.
RESULTS
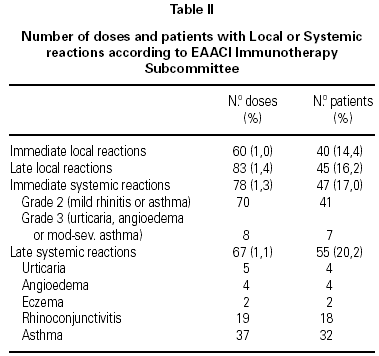
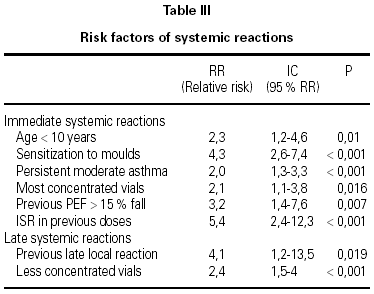
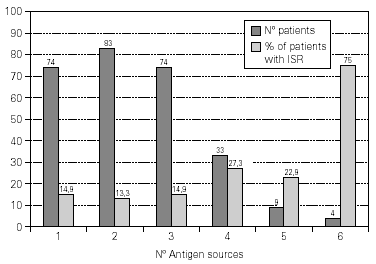
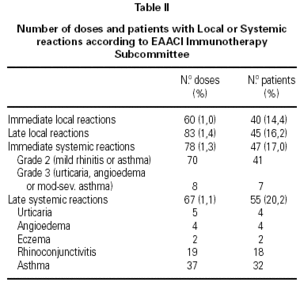
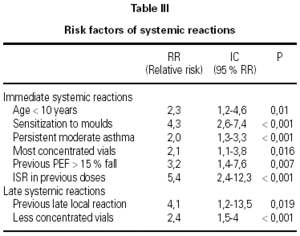
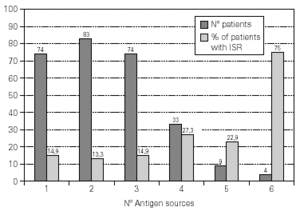
A total of 5,768 administered doses to 273 patients (122 men and 151 women) were analysed. 27.07 % (75 patients) were younger than 10 years old and they received 1469 doses. 3371 doses belong to the build-up phase and 2,397 to the maintenance therapy (table I). 177 were aqueous extracts (hymenoptera venom), 3,973 adsorbed vaccines in aluminium hydroxide, and 1,618 allergoids. All patients but 9 (5 due to local reactions and 4 to systemic reactions) reached the maximum preestablished dose. The number and type of registered adverse reactions are exposed in table II. Only 1 patient presented both types of systemic reaction (immediate and late) after different doses of an Alternaria alternata extract. Most of the immediate systemic reaction (ISR) occurred significantly with the most concentrated vial, in more severe asthmatic patients, in those sensitized to moulds and in children (< 10as). Otherwise, we found that an asymptomatic decrease of more than 15 % in PEF 30 minutes after a dose, showed a significative higher risk of suffering an ISR in the following dose (table III). Also, the presence of an ISR in a previous dose highly increased the probability of being repeated. Polisensitized patients (to more than 3 antigens) showed a higher probability of developing an ISR (p < 0,05) (fig. 1). As an interesting fact we found, according to age, that children younger than 10 years old sensitized to animal dander or with a > 15 % fall in PEF in the previous dose, highly increased the risk of ISR.
Figure 1.--Percentage of patients that show immediate systemic reactions in accordance with the number of allergenic sources to whom they are sensitized: Pollens (gramineae and/or trees, no Cupressaceae), Cupressaceae, Weeds, Mites, Moulds, Epithelia or Hymenoptera.
In relation to the late systemic reactions (LSR), the higher risk was related to the least concentrated vials (increasing dose regimen), and to the presence of another LSR (p = 0.07) or a late local reaction (LLR) in the previous dose.
DISCUSSION
In some researches that employ similar doses regimen and method to ours the RS incidence is smaller than the one we found (2.53 % of the doses)26-28. This fact could be due to, among other factors, that our series include many mould vaccines that have demonstrated, especially in children, to be worse tolerated29,30. On the other hand it is possible that asthma severity in our asthmatic patients was as a whole greater than in another studies. The use of maximum doses with some extracts in which the optimum doses adapted to the patient are not defined could also collaborate to the increase of SR15. However the percentage of patients (36,6 %) who show some SR agrees with Abramson et al review31.
Neither the ISR treated by us nor the LSR showed by our patients were serious, responding all of them to conventional treatment so that we consider that the AIT was safe in our patients.
The risk factors to suffer from RS found in our population, as asthma severity or patients' polisensitization grade, are in agreement with those found by other authors29. The population that we attend comes from a residential area nearby Madrid, with a continental climate that stands out two pollination sharp points (February for cupressaceae and May-June for grass and olive tree pollen), as well as a great proportion of one family-houses and the presence of pets in 50 % of these residences. This could explain that the collective that suffers more from systemic reactions have been housekeepers, exposed more often to house dust mites, moulds and animal dander. This collective is frequently immigrant population that fulfil the treatments worse, and, particularly for asthma, this is usually worse controlled.
We have found that a decrease of more than 15 % in PEF after a dose is a risk factor to present an ISR in the following dose. This fact supports the PEF monitorization recommendations of the EAACI and it can be of great profit in children and other patients, that don't express their symptoms properly, which preclude possible modifications in the dose schedule that would not work otherwise.
As well as in previous studies the local reactions didn't predict the ISR, but they predicted the LSR, what allows us to warn patients about it. In that sense the use of premedication, that we don't employ, but that some other authors do32, could hide the warning that suppose to present a LLR, although further researches are needed to clear up this aspect.
According to our safety medical records we don't consider the administration mistakes as a risk factor (RF). We also minimize the possibility of suffering severe systemic reactions contraindicating the AIT administration in patients using betablocking, with severe asthma or very unstable, or with another medical condition that could reduce the survival in case of a systemic reaction.
We don't find as a RF the administration dates because of the high number of doses administered during the pollinic season. It is possible that clinical stabilization of patients could reduce the risk of reactions throughout these times.
In our patients, in agreement with another researches27,33 the most concentrated vials provoked more ISR, which we treated immediately in our unit. The least concentrated vials provoked more LSR that usually the patient suffered at home. Dose-schedules that reject first doses34 could reduce the LSR incidence without increasing the ISR, though further investigations are needed to assure this possibility.
We agree with Tabar et al26 with the idea that the way of obtaining information in this research must be a prior objective in any Allergy Unit, in order to reduce the incidence of adverse reactions with AIT and to better inform patients. We also agree with the idea that the AIT administration by specialized staff with strict medical records (we don't register neither severe reaction nor anaphylactic shock) is safe enough to exclude any patient in which it is indicated.
We have found as unavoidable RF: sensitisation to moulds or age (children); but other factors such as the PEF variation, ISR or LLR in previous doses that could allow modifying the dosage regimen and reducing adverse reactions; and in every case to inform our patients.
ACKNOWLEDGMENTS
We thank the nurses of our allergy service: Isabel Delgado, Ángela Álvarez, Dionisia Álvarez, Inmaculada Llanos and Kati Rengifo for their help in this study and everyday.