Primary immunodeficiency diseases (PIDD) are associated with significant morbidity and mortality and result in a significant public health burden. This is in part due to the lack of appropriate diagnosis and treatment of these patients. It is critical that governments become aware of this problem and provide necessary resources to reduce this impact on health care systems. Leading physicians in their respective countries must be supported by their own governments in order to implement tools and provide education and thus improve the diagnosis and treatment of PIDD. The Latin American Society of Primary Immunodeficiencies (LASID) has initiated a large number of activities aimed at achieving these goals, including the establishment of a PIDD registry, development of educational programmes and guidelines, and the introduction of a PIDD fellowship programme. These initiatives are positively impacting the identification and appropriate treatment of patients with PIDD in Latin America. Nevertheless, much remains to be done to ensure that every person with PIDD receives proper therapy.
Patients with primary immunodeficiency diseases (PIDD) most commonly present with recurrent bacterial infections, although some may also be affected by systemic and organ-specific autoimmunity, chronic inflammation, and higher susceptibility to certain types of cancer.1 The treatment of most primary immunodeficiencies with impaired antibody production involves immunoglobulin replacement therapy (IRT), either via the intravenous (IV) or subcutaneous (SC) route. These interventions have been demonstrated to be highly effective for the prevention of bacterial and viral infections and reduction of complications and sequelae.2–8 In addition, IRT is effective as adjunctive therapy in the management of selected neurological, haematological, and autoimmune disorders.9–13
Not all PIDD require immunoglobulin therapy replacement. However, antibody deficiency disorders represent up to 60% of all PIDD and appear to be the most common forms in Latin America, based on results from the Latin American Society of Primary Immunodeficiencies (LASID) registry.14 These disorders require IRT. Other types of PIDD affecting antibody production, such as severe combined immunodeficiency (SCID) and other well-defined medical situations, including immunosuppression after stem cell transplantation, may also require immunoglobulin administration. Thus, approximately 85% of all PIDD have some form of antibody deficiency and are therefore eligible for IgG replacement therapy. Guidelines for appropriate use of IgG therapy have been published for Brazil15 and are in advanced-phase preparation for all LASID countries. Effective management of patients with PIDD requires accurate diagnosis and prompt delivery of the appropriate therapy. However, this is not achieved in a substantial percentage of patients. It has been estimated that more than 180 unique genetic diseases are associated with various immunological defects currently defined as PIDD by the International Union of Immunological Societies’ (IUIS) expert committee for PIDD.16 The diagnosis of many of these conditions is often delayed, or patients are misdiagnosed.17–19 Results from one national registry indicated that the interval between the onset of clinical symptoms and diagnosis was significant even in PIDD such as IgA deficiency, which is relatively easy to diagnose.20 There is also evidence suggesting that PIDD are undertreated even after accurate diagnosis. Results from a recent US survey carried out by the Immune Deficiency Foundation (IDF) indicate that approximately 250,000 people in this country have the diagnosis of a PIDD; the majority are antibody deficiencies, either alone or in combination with other immune defects. In each of these PIDD, intravenous immunoglobulin (IGIV) therapy is the standard of care. However, only 22% of the patients surveyed were receiving this treatment.21 These results indicate that increased physician and patient education are needed to improve both the diagnosis and effective treatment of PIDD. Results from small-scale studies have shown that educational interventions can improve both diagnosis and referral to specialists.22–24 Historically, diagnosis and appropriate treatment of PIDD in Latin America has been hampered by a lack of resources and educational opportunities for healthcare professionals. However, a wide range of new initiatives developed and/or supported by LASID is addressing these gaps.
The Latin American Group for Immunodeficiencies (LAGID) was created in 1993 to study the prevalence of PIDD in Latin America and to promote awareness of these diseases. In 2009 this group evolved into the society now named LASID, and created an advisory board that has published four reports and proceedings.25–28 The first two papers focused on the prevalence and characteristics of patients with PIDD in Latin America,25,26 while the third and fourth summarised shortfalls in PIDD diagnosis and treatment in Latin America and described the features of an educational outreach programme, an immunology fellowship programme, and a laboratory network aimed at closing these gaps.27,28
This paper summarises the proceedings from a recent meeting that brought together the members of the LASID advisory board and experts from other Latin American countries and the United States. The aim of said meeting was to consider and discuss the following topics related to the diagnosis and treatment of PIDD in Latin America:
- •
What resources are available to assist physicians in the diagnosis of PIDD?
- •
What are current policies regarding governmental support for immunoglobulin treatment in patients diagnosed with PIDD?
- •
What support and advocacy groups exist in Latin America to assist patients with PIDD, and how can patients and physicians work together to improve diagnosis and treatment of PIDD?
- •
What role should the industry play in improving the diagnosis and treatment of PIDD in Latin America?
This paper also summarises actions that have been initiated to address these issues.
Resources available to physicians for the diagnosis of PIDDAdvancing the diagnosis and treatment of PIDD requires improving physician training and providing better resources for those currently in practice. At present, many specialists who should be concerned about PIDD pay relatively little attention to these diseases. For example, a survey of allergists and immunologists in Brazil indicated that only 5% devoted more than 50% of their practice to PIDD and that 70% devoted less than 10% of their total practice to the diagnosis and management of these patients. Furthermore, over 50% never followed patients that needed immunoglobulin replacement.29 This problem is being addressed by the establishment of the LASID Online Registry programme.14 This programme also includes an educational initiative, as physicians have the opportunity to receive education about PIDD diagnosis before registering patients. Additional educational programmes for healthcare professions have been proposed and initiated by LASID, including a continuing medical education (CME) programme focused on PIDD warning signs, a LASID fellowship programme, a Latin American laboratory network, summer school programmes, and LASID scientific meetings.14
LASID has also taken a lead role in promoting the importance of training in PIDD for allergists/immunologists, pulmonologists, otolaryngologists, rheumatologists, and other subspecialists. A fellowship programme aimed at improving expertise among these groups of physicians has been developed and will be launched in 2012. Subspecialty-specific warning signs for the presence of PIDD are being developed for wider distribution, and it is anticipated that these will be published in 2012. It is also possible to introduce basic information about PIDD into the undergraduate medical curriculum, as this provides a better background for more advanced training of paediatricians and other specialists. LASID is considering topics that should be covered by PIDD training programs and will be directing efforts toward the recruitment of Latin American countries that have not participated in programmes to date. Thus, in addition to awareness programs, LASID delivers education, research, registry, and fellowship programmes, and is currently editing IGIV therapy guidelines for Latin America.
A number of additional educational initiatives that have the potential to increase the diagnosis of PIDD have been undertaken:
- •
Agencies in Brazil (Fundação de Amparo a Pesquisa do Estado de São Paulo, Conselho Nacional de Desenvolvimento Científico e Tecnológico) have supported research programmes in PIDD, including educational efforts emphasising that PIDD are well characterised and are not “unknown” diseases. This approach has proved to be effective in a pilot programme aimed at improving newborn and PIDD screening for those with adverse reactions to bacillus Calmette-Guérin (BCG) vaccination in São Paulo.30
- •
Another programme developed and implemented in Brazil has the potential to improve diagnosis of PIDD by both primary care practitioners and specialists who have limited experience with these diseases. A set of cards describing symptoms and laboratory abnormalities that should raise suspicion of PIDD among different specialists has been developed and distributed. It is important to continue supporting these physicians with discussions of clinical cases using the Internet. In addition, as noted above, a paper is in development that provides detailed information regarding warning signs for PIDD that might be seen by non-immunologist physicians.
- •
In Colombia, an initiative similar to that described above has been in place for more than five years. This initiative includes a national educational programme that has provided PIDD training in more than 150 hospitals throughout the country, with more than 4000 people attending. Supporting materials, such as cards containing algorithms for PIDD diagnosis and normal values for serum immunoglobulins and lymphocyte subsets in the general population, have been distributed among healthcare practitioners. In addition, a pilot programme called IDPnet has been established to improve diagnosis of PIDD. With this programme, hospitals from four different regions are being provided with funding by the Colombian Primary Immunodeficiency Foundation, and the industry (Baxter) to cover additional expenses for diagnosing PIDD patients—including patients in a national registry—and training physicians to improve local resources. This is an important advancement because once patients are properly diagnosed as a result of this programme the government is required by law to provide the proper treatment throughout a health care system that partially subsidises these treatments.
- •
Chile undertook an educational programme in 2002 to improve the diagnosis and treatment of PIDD. A fellowship programme in allergy-immunology was begun in 1974 and, starting in 1988, gave more emphasis to PIDD. Other fellowships in paediatric infectious diseases and paediatric pulmonology have included training in PIDD. As a result of this programme, 12 out of 15 regions in Chile now have coverage for immunology, and there is a strong commitment to diagnosis and treatment of PIDD.
- •
In Mexico, the Mexican Foundation for Children with Primary Immunodeficiencies with the support of an unrestricted grant from all major immunoglobulin producers (CSL Behring, Baxter, Grifols, Octapharma, and Talecris), has implemented a workshop for first-contact physicians (general practitioners and paediatricians) to teach them when and how to evaluate children with recurrent infections. To date, more than 2000 physicians have participated in the workshop. This effort resulted in an immediate increase in referrals to the Mexican Jeffrey Modell Diagnostic Center at the National Institute of Pediatrics. It also consolidated the Mexican network for diagnosing PIDD. In addition, the health ministry has supported up to two years of additional advanced PIDD training for fellows after they finish a paediatric allergy and immunology programme. This programme includes the opportunity for a one-year research experience.
- •
In Argentina, immunology is not recognised as a specialty by the Public Health Ministry, but efforts are ongoing with the Argentinian Immunology Society to change this situation. Two hospitals in Buenos Aires (Hospital Ricardo Gutierrez for Children and the JP Garrahan Hospital) offer medical and biochemical residency programmes and provide scholarships of one to two years for projects in an immunology speciality funded by government agencies. Postgraduate immunology programmes are not available in other parts of the country. Since 2004, the Immunology Unit of the Hospital Ricardo Gutierrez has offered an annual postgraduate course titled “The Immunology of Today: From Molecular to the Clinic.” This year the course is going to be delivered via the Internet to 220 participants. In addition, physicians from the Children Hospital Ricardo Gutierrez travel from Buenos Aires to the provinces to provide information and courses about different immunological diseases to physicians and laboratory professionals.
- •
The LASID website was launched recently (www.lasid.org) and will post educational and consensus materials. This has been done successfully by the Brazilian Group of Primary Immunodeficiencies for several years (www.bragid.org.br).
Concerted efforts are needed to promote increased support for the treatment of PIDD in Latin America. A number of topics should be considered:
- •
Gaining increased support for IRT requires a key focus on governmental agencies. Under the Universal Declaration of Human Rights, it must be made clear to every government that to be healthy is a basic human right and that the establishment of diagnostic and treatment centres for PIDD is essential to cover this neglected patient population.
- •
It is important for every Latin American country to collect local data regarding the incidence and prevalence of PIDD and the burden and impact of these diseases in their respective countries; this will enable them to secure the appropriate support of advocacy initiatives by local government leaders. As noted above, the LASID Online Registry has already provided a convenient vehicle for the development of epidemiological information for all of Latin America, as well as for individual countries. It is important to understand that governments are always looking for ways to provide the best health care at the lowest cost. Therefore, it is important to support initiatives directed toward the collection of accurate information that effectively compares the cost of IRT versus the costs for untreated PIDD and its associated complications.24 The economic burden of treatment is high, and it is essential to convince government officials that it is more expensive to provide care for patients with undiagnosed PIDD than it is to diagnose and effectively treat the condition. The benefit of IRT for decreasing hospitalisation, physician visits, and school absences for children with PIDD is well documented.31
- •
It needs to be remembered that there are distinct differences among cultures and approaches to healthcare management in different Latin American countries. It is essential to keep these differences in mind when addressing health economic issues; different strategies might be required to achieve governmental support in each country. Specifically, while the engagement of politicians may be effective in some countries, it may not be in others. For example, in Costa Rica, the constitutional court may be the best venue to seek support for IRT for patients with PIDD. Costa Rica's social security system determines whether or not a medication for a patient with a specific diagnosis will be reimbursed. If the social security system does not provide the medication, the case is brought before the constitutional court, which has the power to order the social security system to pay for medications. In Colombia, the health care system is tightly bound to what appears to be a fair approach for improving access to these types of medications. Furthermore, with the passing of a rare diseases act in August 2010, Colombia now mandates appropriate medication for patients with PIDD, among other rare diseases. However, these types of initiatives are expensive and often fall short when economic resources are scarce, leaving patients without coverage.
- •
A LASID survey of reimbursement for IRT in Argentina, Brazil, Chile, Colombia, Honduras, and Mexico has indicated differences in coverage. While IRT is covered by the government in Argentina, Brazil, Honduras, and Mexico, in Chile coverage is obtained partially by the public system and fully via the patient's individual insurance in the private system. In Colombia, by contrast, treatment is not covered under the universal health plan, but by a special governmental fund that reimburses health insurance providers who pay for IRT. Thus, while there is apparently appropriate governmental support for PIDD treatment in many Latin American countries, many patients with these conditions are not properly treated. For example, results from a pilot survey in Colombia indicate that only 23.5% of patients with the correct diagnosis of antibody deficiency receive IRT (Orrego JC, personal communication). It is hoped that the LASID-directed revision of PIDD guidelines for Latin America will help to promote governmental and private insurer reimbursement for appropriate treatment of patients with PIDD throughout this region.
Initiatives aimed at gaining governmental support for the diagnosis and treatment of PIDD need to be designed for individual countries, but there are general principles that can be employed to guide these programmes. A first important step is to develop and disseminate authoritative recommendations supporting the appropriate treatment of patients with PIDD. This is being accomplished with the completion of the new Latin American treatment guidelines.
Local information in the form of registries, such as the LASID Online Registry, must be gathered and presented to the relevant authorities most likely to be concerned about the cost of IRT. Such registries collect information related to the incidence, prevalence, and costs of PIDD, as well as the social burden resulting from patients who are not diagnosed or who are diagnosed but untreated. However, this approach requires the commitment of Latin American healthcare professionals to carry out these studies with the support of their respective governments and to provide economic data to emphasise the cost-effectiveness of appropriate PIDD treatment. An analysis of the expense of immunoglobulin treatment in US patients with PIDD, which included costs associated with treatment of infections, hospitalisation, physician visits, and time lost from work, showed approximately $25,300 in health-related savings per patient, which nearly completely offset the cost of immunoglobulin administration.32 It is important to note that this analysis did not attach a monetary value to quality of life, which is a fundamental consideration. Several studies have demonstrated that immunoglobulin treatment significantly improves quality of life in patients with PIDD,33 and this should be a focus of Latin American pharmacoeconomic analyses as well. It is also important that physicians in each country become familiar with the reimbursement system for immunoglobulins and the laws related to it. This knowledge should help guide efforts aimed at gaining payment for appropriate medication in patients diagnosed with PIDD. Here again, dissemination of treatment guidelines is important. New treatment guidelines have been published for Brazil,15 and those for all of Latin America will be available soon.
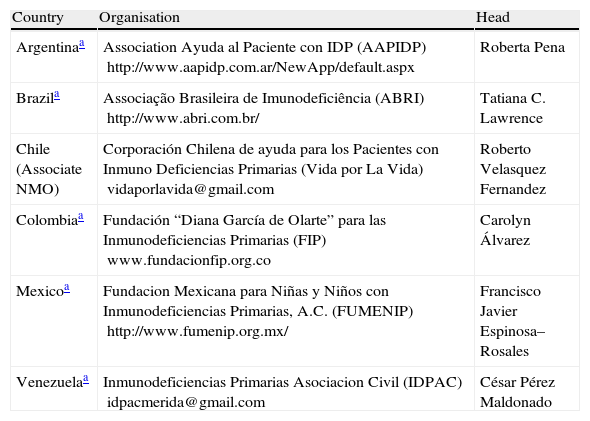
Collaborations between patients and healthcare professionals: support and advocacy groupsPartnering with advocacy groups is becoming a fruitful approach for improving the diagnosis and treatment of PIDD in Latin America. These organisations are developing strategies to communicate about these diseases in order to change the general public's perception about PIDD. For example, case histories that can be provided by advocacy groups (e.g., descriptions of long periods without diagnosis, difficulty in gaining treatment) are important for putting a “face” on PIDD and emphasising its impact on patients and their families. Patient advocacy groups exist in many countries in Latin America (Table 1), and physician organisations concerned with PIDD should partner with them to lobby for improved diagnosis and treatment, as foundations/patient advocacy groups have already established strong relationships with both politicians and healthcare providers. As with initiatives directed at governmental officials, advocacy programmes should be tailored for each country and reflect cultural differences.
PIDD Patient advocacy groups in Latin America.
| Country | Organisation | Head |
| Argentinaa | Association Ayuda al Paciente con IDP (AAPIDP) http://www.aapidp.com.ar/NewApp/default.aspx | Roberta Pena |
| Brazila | Associação Brasileira de Imunodeficiência (ABRI) http://www.abri.com.br/ | Tatiana C. Lawrence |
| Chile (Associate NMO) | Corporación Chilena de ayuda para los Pacientes con Inmuno Deficiencias Primarias (Vida por La Vida) vidaporlavida@gmail.com | Roberto Velasquez Fernandez |
| Colombiaa | Fundación “Diana García de Olarte” para las Inmunodeficiencias Primarias (FIP) www.fundacionfip.org.co | Carolyn Álvarez |
| Mexicoa | Fundacion Mexicana para Niñas y Niños con Inmunodeficiencias Primarias, A.C. (FUMENIP) http://www.fumenip.org.mx/ | Francisco Javier Espinosa–Rosales |
| Venezuelaa | Inmunodeficiencias Primarias Asociacion Civil (IDPAC) idpacmerida@gmail.com | César Pérez Maldonado |
A main advantage of establishing partnerships with advocacy groups is in the organisation and sponsorship of specific events aimed at increasing awareness about PIDD. A good example is the Day of Immunology, established by the European Federation of Immunological Societies (EFIS) in 2005. Its purpose is to raise awareness among the public, press, politicians, and decision makers about the critical importance of the immune system. In 2010, more than 30 countries participated in the event. There is now a PIDD week that leads up to World Immunology Day. On April 28, 2010, in celebration of World Immunology Day, the International Patient Organization for Primary Immunodeficiencies (IPOPI); the Jeffrey Modell Foundation (JMF); the International Nursing Group for Immunodeficiencies (INGID); and the European Society for Immunodeficiencies (ESID) launched a united call to action to offer guidance for governments about the steps they can take to understand, appropriately diagnose, and manage PIDD in their respective countries.34 This call to action focuses on four elements: raising awareness of PIDD, educating health professionals and fostering exchanges in expertise, early diagnosis and screening, and encouraging countries to ensure comprehensive and adequate treatment.34 This call to action was also launched in Latin America during the LASID meeting in Mexico, October 2011 and will enhance LASID activities.
The 2011 Immunology Day was effective in Latin America. In Brazil, a web conference was organised for several Latin American countries (Argentina, Brazil, Mexico, Chile, and Colombia), with topics that included PIDD diagnosis, treatment, bone marrow transplantation, registry, and patient advocacy. In Brazil, a meeting for PIDD patients was held, with approximately 150 people participating. In Colombia, a nationwide initiative was launched to perform an online survey of physicians’ knowledge about the warning signs of PIDD. The aim of the campaign was to question whether patients knew more about PIDD than the physicians themselves, due to the wide availability of information about these diseases that patients have even before they go to the doctor. In Argentina, a full-day symposium covering the same topics took place in Buenos Aires. Programme participation was enhanced by radio and newspaper advertising. In Argentina, the celebration of Immunology Day lasted an entire week (April 25–29) in five hospitals in Buenos Aires. Physicians, patients, and families could attend the play Booster Doesn’t Have Antibodies. In Chile, a symposium organised by the Vida por la Vida Corporation was held in Santiago.
The overall aim of World Immunology Day and related events is to empower and engage global stakeholders in PIDD. These events are designed to facilitate advocacy, and this can best be achieved by including all relevant groups, such as physicians, nurses and other allied health professionals, patients and their national member organisations, and industry. The initiatives provide tools, templates, and branded materials that help physicians and health care providers increase their understanding of the human toll as well as the societal economic burden of PIDD, thus putting a human face on these conditions.
Understanding of the genetic and molecular basis of PIDD is progressing rapidly and opening new avenues for treatment advances and this requires advanced diagnostic capabilities. A growing network of Jeffrey Modell Diagnostic and Research Centers is contributing to develop these capabilities in Latin America, starting with the first Latin American Center in São Paulo in 2007, followed by new centres in Mexico in 2009, Colombia and Chile in 2010, and Argentina in 2011. This network will provide additional expertise and capabilities for the advancement of diagnosis and treatment for PIDD in Latin America.
Role of the industryThe support of the industry for initiatives aimed at increasing awareness of PIDD and improving treatment is vital for achieving success. This support has included funding for the maintenance of patient registries, associated educational initiatives aimed at improving PIDD diagnosis, meetings, and advocacy of the type described above. An important project that would benefit from industry support is the First Summit on Primary Immunodeficiencies for Latin America. This summit took place in Mexico during the 2nd LASID meeting in October 2011. It brought together health ministers from Mexico and Central and South America with physicians and PIDD patients and their families to increase awareness of the human burden of PIDD and to lower barriers to effective treatment. In Latin America, industry support has already been gained for the LASID Online Registry (http://imunodeficiencia.unicamp.br:8080/estatistica_mensal.html), the LASID fellowship programme, the LASID Summer School programme, other educational and research programmes and associated presentations at international meetings, PIDD screening, and LASID meetings.
ConclusionsPrimary immunodeficiency diseases are relatively common and represent a challenge for the health systems in Latin America. It is important that government officials in these countries are made aware of the large numbers of people in their respective countries who are likely to have PIDD. This message needs to be delivered by a strong, united voice that includes both patients and healthcare professionals. Leading physicians in Latin America must reach out to government health officials with effective advocacy aimed at highlighting the importance of proper diagnosis and support for treatment of PIDD patients. A pan-Latin American meeting of health officials, physicians, and patients will be a very effective way to deliver these key messages; however, these interactions must also be accomplished on a country-by-country basis. Success in one country might be used as an example to prompt action in another. Improving the lives of patients with PIDD can only be achieved through strong and efficient partnerships among physicians, governments, industry, and patients. Such partnerships have already resulted in the implementation of a large number of programmes aimed at improving the diagnosis and treatment of PIDD in Latin America, including the LASID Online Registry, the LASID fellowship programme, educational and research programmes, guideline development, and pilot PIDD screening projects. An important goal for LASID over the next several years will be to help establish strong bonds among these stakeholders in Latin America to reduce the barriers to PIDD diagnosis and care.
The authors thank Baxter Biosciences for supporting the Latin American Advisory Board on Primary Immunodeficiencies initiative as well as supporting the editorial services for the development of the proceedings of this initiative.