To the Editor:
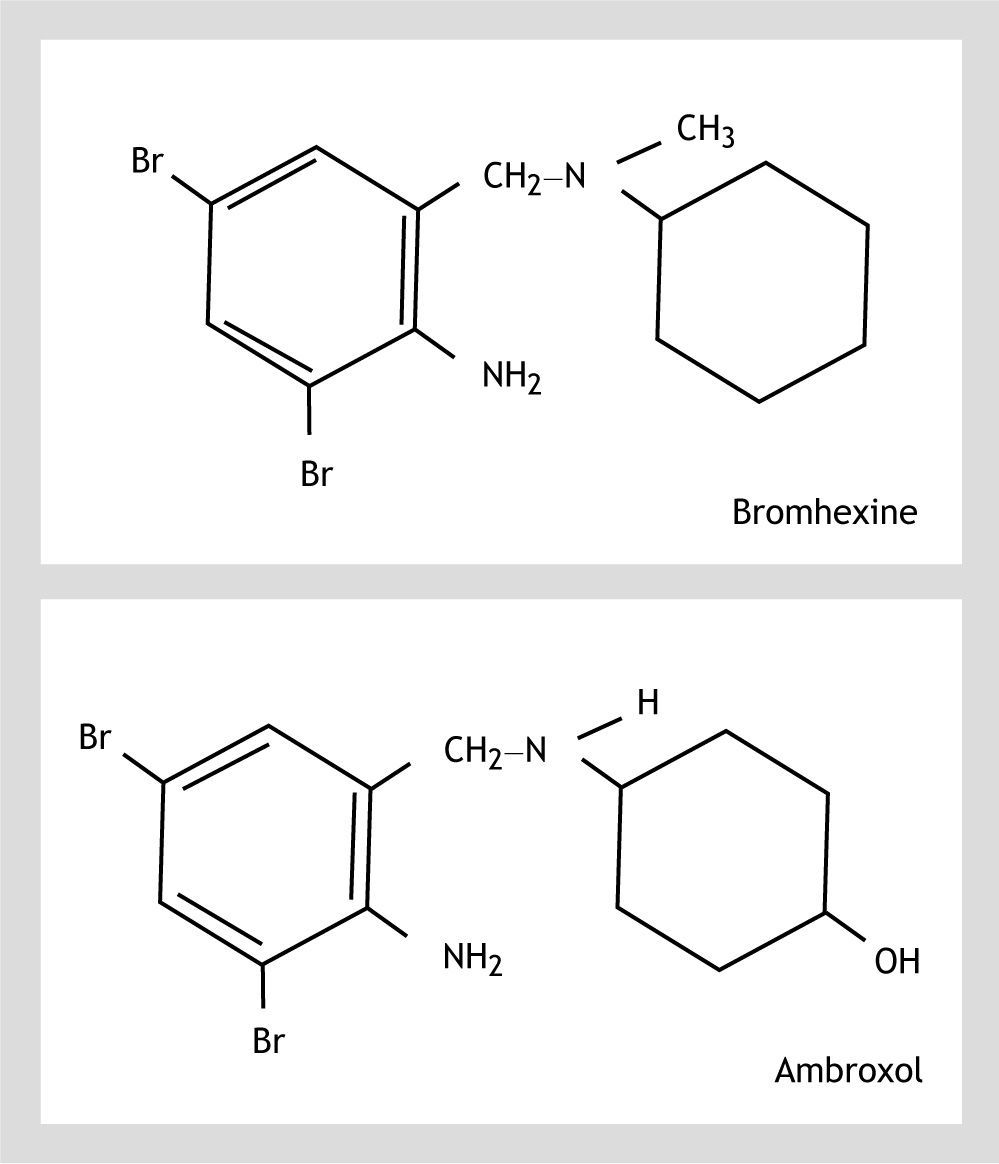
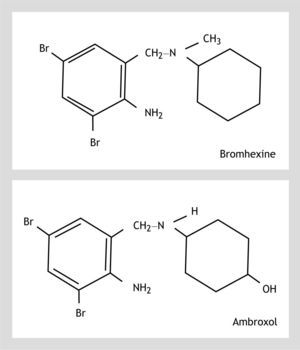
Ambroxol hydrochloride (trans-4-(2-amino-3,5-dibromobenzylamino) cyclohexanol hydrochloride) is a bromexine metabolite1 (Fig. 1). Both ambroxol and bromexine are well known mucolytics and ambroxol has also been used in clinical trials for the prevention of chronic bronchitis and respiratory distress syndrome in infant.
A 79-year-old woman presented a maculopapular rash with intense pruritus. She had taken ambroxol, acetaminophen, and codeine for 4days at doses of 90mg/day, 1,500mg/day and 90mg/day respectively, prescribed for odynophagia. On clinical examination there was a generalised maculopapular exanthematous eruption, with furfuraceous desquamation and intense erythema. The mucosa was spared. She was treated with hydratation, antihistamines, and oral corticosteroids and the skin lesions resolved within a week. After this episode the patient tolerated acetaminophen and acetylsalicylic acid. She had no personal and familiar history of atopic diseases.
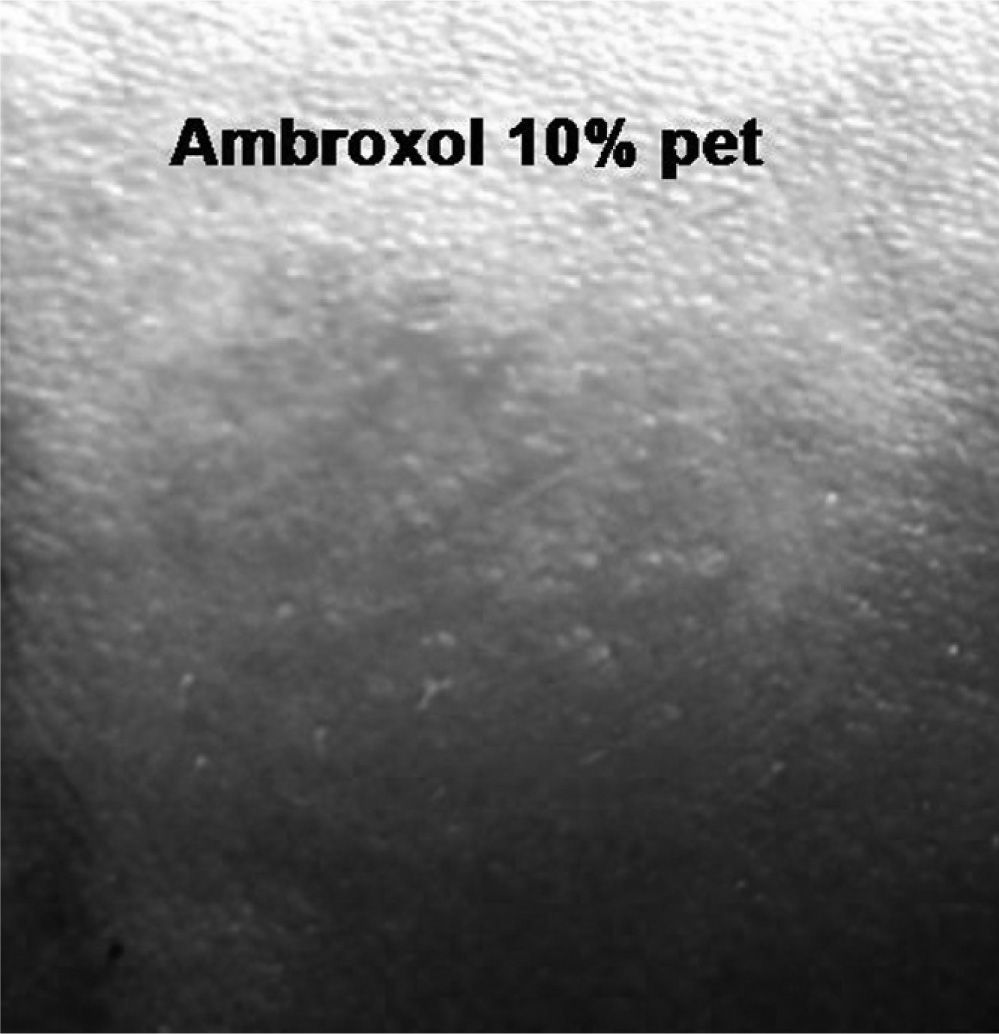
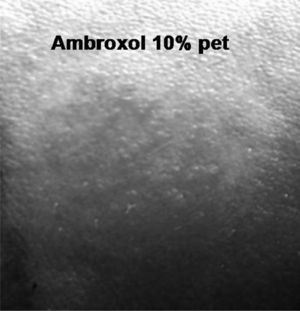
Patch tests were performed, according to the guidelines of the International Contact Dermatitis Research Group, with the GEIDC standard series, ambroxol (10 % pet) and codeine (10 % pet).
Positive reaction was found to ambroxol at 48 hours (++) which increased at 96 (+++) (Fig. 2), and negative to codeine and the standard series. Patch tests were negative in 10 controls. The oral challenge was not carried out for ethical reasons.
Delayed reactions to systemic drugs are not rare and patch test is a well-known method for diagnostic confirmation pur-posed in patients with generalised type IV hypersensitivity reactions.1,2 We report a systemic contact dermatitis due to ambroxol, presented as a maculopapular rash and confirmed by patch testing.
Systemic cutaneous reactions by immediate hypersensitivity have been described during the treatment with ambroxol such as urticaria. Delayed reactions to ambroxol such as non-pigmented fixed erythema3 and contact sensitivity to ambroxol have also been reported when administered by aerosol,4 but as far as we know, this is the first case of systemic generalised dermatitis caused when taken orally.
Exanthema induced by bromexine has been reported5 and despite the chemical structures being very similar, the absence of cross-reactivity between them has been demonstrated in one case.4 More studies should be reported to as certain it definitely. We did not perform an oral challenge with bromexine because of the possibility of inducing a severe reaction, and because it was not an essential drug for the patient.