Although some studies show that IL-22 and IL-25 play critical roles in the pathogenesis of asthma, little is known about the systemic production of these cytokines. The aim of this study was to assay IL-22 and IL-25 in serum, in mitogen-activated whole blood (WB), and in mitogen-activated peripheral blood mononuclear cell (PBMC) cultures of patients with severe asthma.
MethodsIn this cross-sectional study, a questionnaire was prepared to determine the severity of asthma. Through the questionnaire, information including clinical signs, clinical symptoms, and past medical history were acquired. Information collected allowed all patients who were active or ex-smokers to be excluded. A trained observer assessed airway reversibility, peak flowmetry, and spirometry in the remaining patients. Twenty-one patients and simultaneously, twenty age- and sex-matched healthy controls were selected. Sterile blood (10ml) was taken from each study participant. Sera were isolated and anticoagulant blood used for WB and PBMC cultures and haematological tests. Phytohaemagglutinin and lipopolysaccharide (LPS) were used to activate WB and PBMC. The data from these two groups were compared using Student's t-test.
ResultsAlthough the total white blood cell count was elevated in the asthmatic group, other haematological indices, including IL-22 and IL-25 levels in the asthmatic group were not significantly (p>0.05) different from controls.
ConclusionsThe levels of IL-22 and IL-25 in patients with severe asthma are no higher than those of non-asthmatic individuals. Any major role for IL-22 and IL-25 in severe asthma is likely to be localised to the lungs and bronchial tissues.
Bronchial asthma is a common and chronic inflammatory disease of the lung, characterised by partially reversible obstruction of the lower respiratory tract, increased bronchial responsiveness for several stimuli, and airway inflammation.1,2 Allergic airway inflammation is characterised by eosinophil and lymphocyte infiltration, inhibition of neutrophil infiltration, elevated serum IgE level, airway remodelling, and airway hyperreactivity. Some studies show that airway inflammation in bronchial asthma is initiated by response of Th2 subset of T cells, which mainly produce cytokines such as IL-4, IL-5 and IL-13.2–4
Besides IL-4, IL-5 and IL-13, some investigations have shown that IL-22 and IL-25 may have pivotal roles to play in inflammation and asthma. IL-22 is a cytokine that is important for the modulation of tissue response during inflammation and plays an important role in inflammatory diseases.5 IL-22 belongs to the IL-10 family of cytokines. One of the major sources for IL-22 production is the Th17 subset of T cells. The receptor for IL-22 is mainly expressed on epithelial cells of the digestive and respiratory systems, and in keratinocytes in the skin. IL-22 induces expression of some antimicrobial peptides and proteins that cause differentiation and survival of these cells.2,6–8 In addition, IL-22 protects lung function by increasing transepithelial resistance to injury and promotes induction of epithelial cell proliferation.9 Neutralisation of IL-22 has been shown to augment eosinophil recruitment to the lung and increase allergic response.10
IL-25 (or IL-17E) is a member of the IL-17 family of cytokines. This family contains six structurally related, but functionally distinct, proteins: IL-17A to IL-17E.11 IL-25 seems to induce IL-4, IL-5 and IL-13 from Th2 and mast cells and plays an important role in the maintenance of Th2 type immune response and contributes to the pathogenesis of asthma.3,11 T cells, lung epithelial cells, mast cells, basophils, and eosinophils may produce IL-25.12
To our knowledge, most of the related investigations that indicate IL-22 and IL-25 might play a role in the pathogenesis of asthma have been performed on respiratory tissues. Increased levels of IL-222,13 and IL-2511,13,14 have been found in bronchial biopsies and bronchoalveolar lavage11 fluids from patients with asthma. Meanwhile, there also exists some controversial data about the serum IL-22 and IL-25 levels in asthmatics15,16 and scant data concerning the levels of these two cytokines in the supernatant of whole blood (WB) and within peripheral blood mononuclear cell (PBMC) cultures activated by mitogens. Therefore, the aim of the study was to assay levels of IL-22 and IL-25 in serum, in mitogen-activated WB, and in mitogen-activated of PBMC cultures from patients with severe asthma.
Materials and methodsPatientsThis cross-sectional study was conducted in the pulmonology clinic of Shahid Beheshti Hospital affiliated to Hamadan University of Medical Sciences, Iran. Ethical approval was achieved from the Ethics Committee of Hamadan University of Medical Sciences and written informed consent was obtained from all participants. Twenty-one patients with severe chronic asthma, before treatment or previously diagnosed, who had not received any drugs within the past four weeks, were chosen. The inclusion criterion for all cases was bronchial asthma, where the diagnosis was established through demonstrating reversible airway obstruction and were diagnosed with severe and persistent asthma (severity step 4) according to the Global Initiative for Asthma criteria.17 Study participants completed a questionnaire of their demographic characteristics, including age, sex, asthma history, past medical history, and details related to current asthma exacerbation, nocturnal, and diurnal clinical signs and symptoms. To identify the severity of asthma, a trained observer assessed airway reversibility, peak flowmetry, and spirometry in the asthmatic patients. At least three acceptable manoeuvres meeting the American College of Chest Physicians Standards were required, with the minimum of two reproducible forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) manoeuvres within 5% of best required for each test.18
The airway responsiveness was performed according to a standardised protocol.19 Airway reversibility was evaluated by spirometry before albuterol treatment and again 15min after inhalation of two puffs of a beta-adrenergic agonist (albuterol) via a metered dose inhaler. An increase of 12% or more in FEV1 following albuterol treatment, and by at least 200ml, was considered diagnostic for asthma.19 Peak expiratory flow (PEF) was also used to assess acute asthma severity and was expressed as percentage of the value based on age, sex, race, and height. Changes in PEF are expressed as the relative changes in percentage of the predicted value.
The exclusion criteria were the presence of any inflammatory diseases and history of recurrent infections, viral hepatitis, known collagen vascular diseases, autoimmune diseases, chronic obstructive lung disease (other than bronchial asthma), myocardial infarction, unstable angina, and having been under any surgical procedures during the previous month. Patients who previously used inhaled corticosteroids or systemic steroids within the past four weeks and those who were active smokers were excluded from the study.
In a similar way, twenty age- and sex-matched healthy and unrelated control subjects with no personal or family history of asthma and other inflammatory disease, were recruited from the same geographical area through a blood donor clinic.
Blood collectionSterile blood samples (10ml) were taken from each patient and control. Blood samples were collected in two specimen containers, one containing ethylenediamine tetraacetic acid (EDTA) as anticoagulant, and the other without anticoagulant. Sera were isolated and frozen at −80°C for subsequent ELISA analysis, and anticoagulant blood used to culture WB and PBMCs, and haematological tests.
Haematological testsTotal and differential white blood cell (WBC) counts, red blood cell counts, haemoglobin, haematocrit, platelets, and the ratio of neutrophils/lymphocytes of the samples were determined using standard laboratory procedures and an MS9 cell counter analyser (Melet Schloesing MS9; Cergy Pontoise, France).20
In vitro mitogen-activated production of IL-22 and IL-25 by WB cell culturesTo determine the in vitro production of IL-22 and IL-25, one ml of fresh blood containing anticoagulant was suspended in one ml complete RPMI1640 medium (Gibco-BRL, Australia) containing 100U/ml penicillin G (Hayan, Iran), 10% FCS (Gibco-BRL, Australia), 100μg/ml streptomycin (Hayan, Iran) and 5μg/ml Phytohaemagglutinin21 (Sigma, Germany), 25μg/ml lipopolysaccharide (LPS) (Sigma, Germany), and incubated for 48h in a CO2 incubator at 37°C. Thereafter, the supernatants were collected and frozen at −80°C for subsequent cytokine assays.22,23
In vitro mitogen-activated production of IL-22 and IL-25 by PBMC culturesBriefly, 4ml of fresh blood containing anticoagulant was diluted with 8ml of Hank's solution. PBMCs were isolated by Ficoll-Paque and washed twice with Hank's solution. From these PMBCs, 7×105 cells were cultured as a monolayer culture in 1ml of RPMI1640 medium, supplemented with materials as per the procedure above for WB cells, and incubated for 48h in a CO2 incubator at 37°C. The supernatants were collected and frozen at −80°C for future cytokine assay.22
Cytokine measurement by ELISASerum and supernatant levels of IL-22 and IL-25 were determined by sandwich enzyme-linked immunosorbent assays according to the manufacturer's instructions (Koma Biotech, Seoul, South Korea). All assays were carried out in duplicate. The standard curves ranges for the kits were 16–1000pg/ml and were generated by Smart Magellan™ data analysis software.
Statistical analysisResults were expressed as mean±standard error. Unpaired Student's t-test was used to compare the means of the examined groups. All comparisons were two-sided with P values given for each group to indicate statistical significance. The statistical software used for this analysis was SPSS version 16.
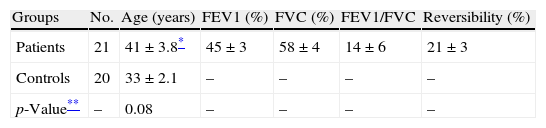
ResultsTwenty-one patients with asthma and 20 healthy controls were enrolled in this study; the data in Table 1 show diagnostic parameters used to select the severe chronic asthmatic patients who were at step 4 of disease severity.
Diagnostic parameters in patients with asthma.
| Groups | No. | Age (years) | FEV1 (%) | FVC (%) | FEV1/FVC | Reversibility (%) |
| Patients | 21 | 41±3.8* | 45±3 | 58±4 | 14±6 | 21±3 |
| Controls | 20 | 33±2.1 | – | – | – | – |
| p-Value** | – | 0.08 | – | – | – | – |
FEV1, forced expiratory volume in the first second; FVC, forced vital capacity.
All the patients were diagnosed as in step 4 of disease according to the Global Initiative Asthma protocol.
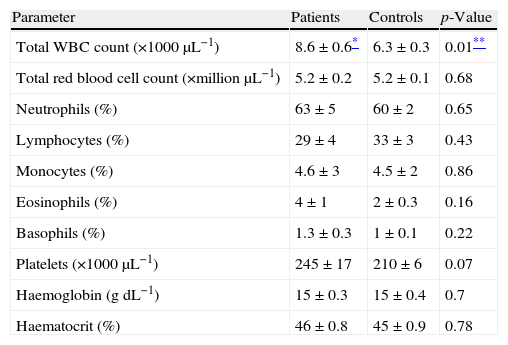
The mean value of the total WBC count was increased in the patients with asthma (8600/μl) in comparison with the controls (6300/μl). Nevertheless, the mean values of total red blood cell counts, differential count of neutrophils, lymphocytes, monocytes, eosinophils and basophils, platelet counts, haemoglobin and haematocrit were not significantly (p>0.05) different in the asthmatic patients in comparison with the control group (Table 2).
Comparison of the haematological indices from the control group and the asthma patients.
| Parameter | Patients | Controls | p-Value |
| Total WBC count (×1000μL−1) | 8.6±0.6* | 6.3±0.3 | 0.01** |
| Total red blood cell count (×million μL−1) | 5.2±0.2 | 5.2±0.1 | 0.68 |
| Neutrophils (%) | 63±5 | 60±2 | 0.65 |
| Lymphocytes (%) | 29±4 | 33±3 | 0.43 |
| Monocytes (%) | 4.6±3 | 4.5±2 | 0.86 |
| Eosinophils (%) | 4±1 | 2±0.3 | 0.16 |
| Basophils (%) | 1.3±0.3 | 1±0.1 | 0.22 |
| Platelets (×1000μL−1) | 245±17 | 210±6 | 0.07 |
| Haemoglobin (gdL−1) | 15±0.3 | 15±0.4 | 0.7 |
| Haematocrit (%) | 46±0.8 | 45±0.9 | 0.78 |
All comparisons were two-sided.
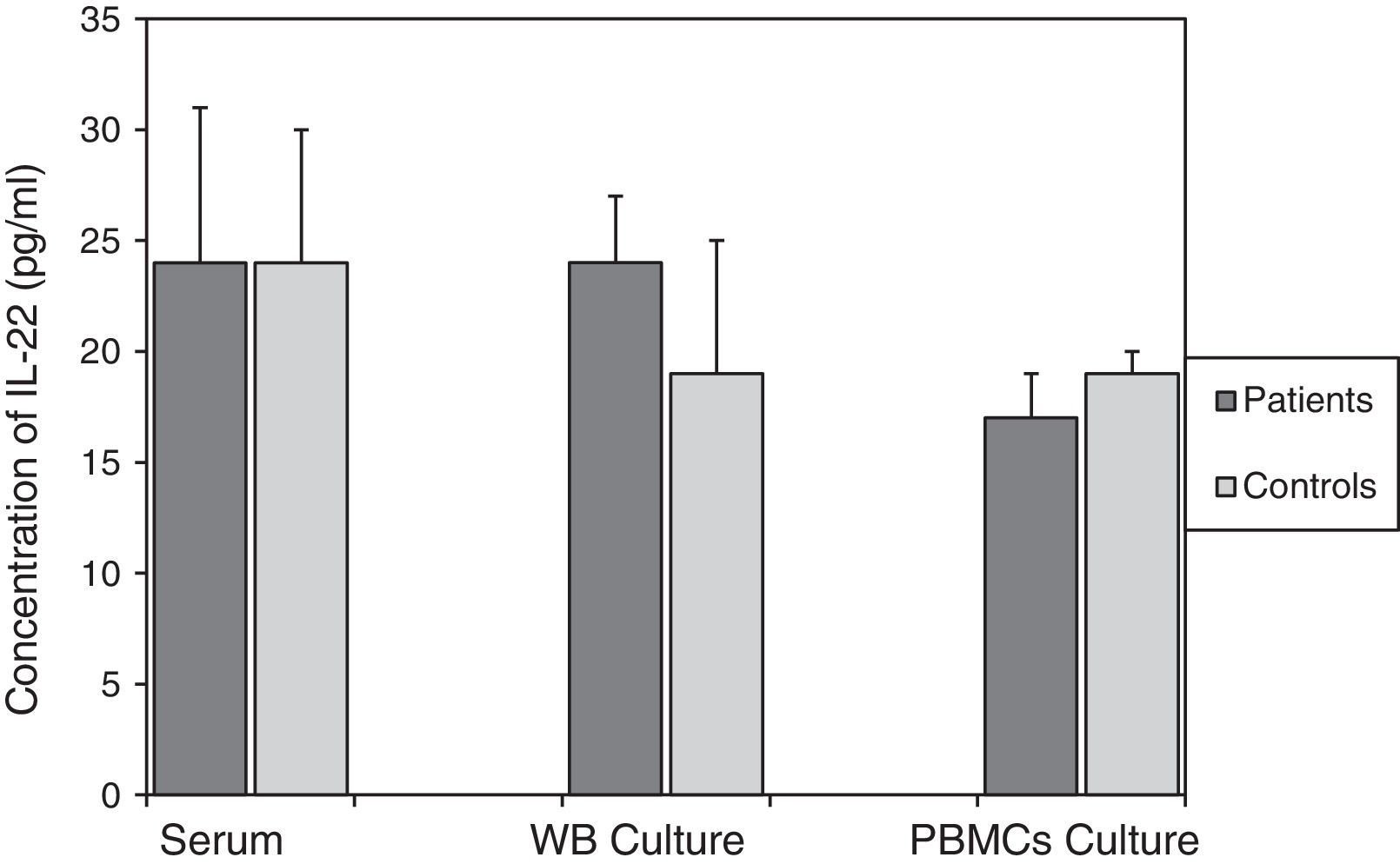
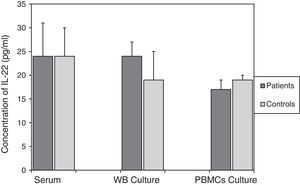
For asthmatic patients, levels of IL-22 in serum (24±6pg/ml), the culture supernatant of mitogen-activated WB (24±6pg/ml), and in the culture supernatant from mitogen-activated PBMCs (17±1pg/ml) were not statistically different from those found in the control group. The values of which were, respectively, 24±7pg/ml; p=0.982, 19±3pg/ml; p=0.446, and 19±2pg/ml; p=0.354 (Fig. 1).
Comparison of IL-22 levels in samples taken from patients with asthma and the non-asthmatic control group. There were no statistically significant differences between serum IL-22 levels (p=0.982), or levels of IL-22 in the supernatants of WB (p=0.446) and PBMC (p=0354) cultures activated by mitogens PHA (5μg/ml) and LPS (25μg/ml), in the asthmatic patients and controls. Data are presented as mean±SEM.
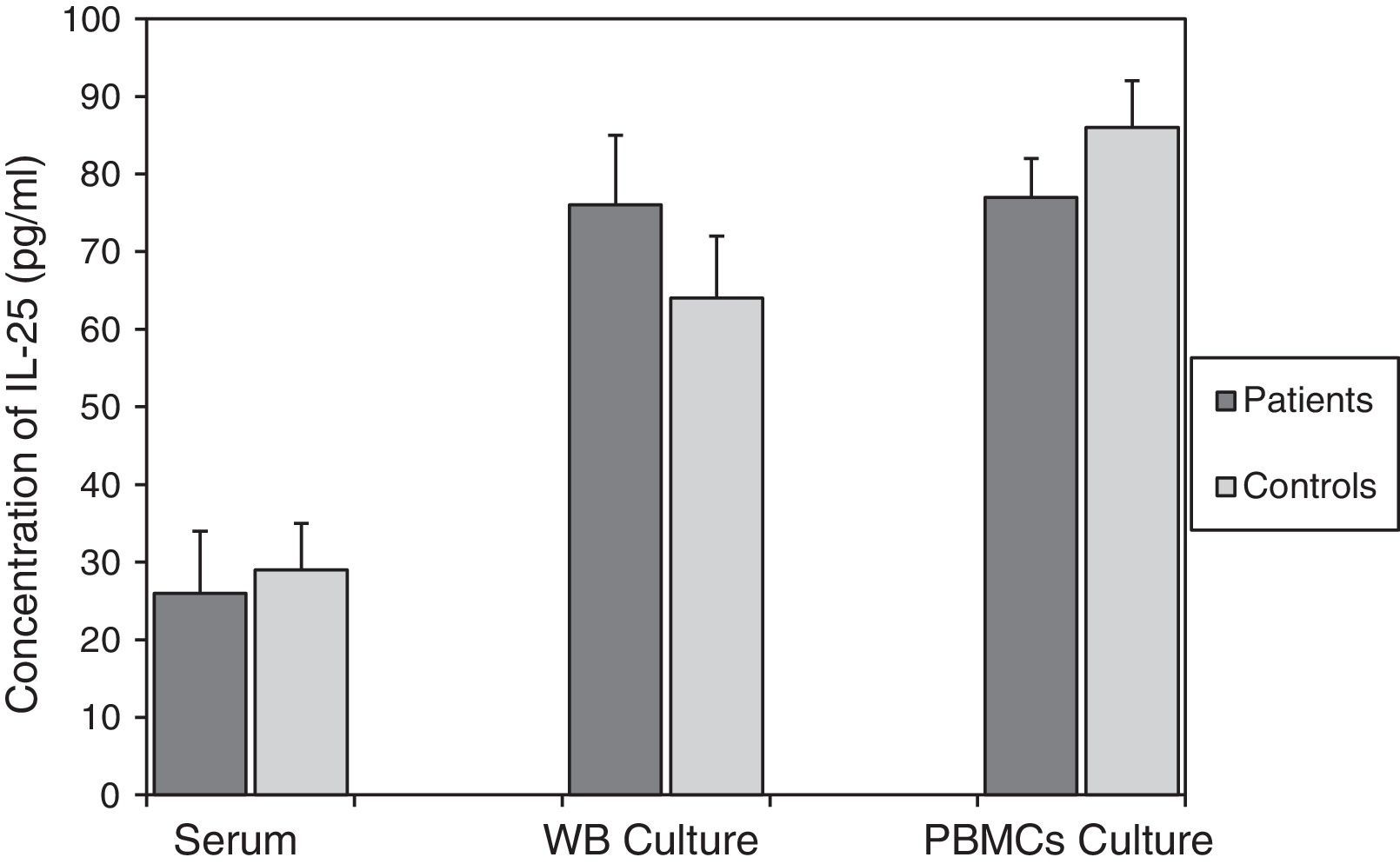
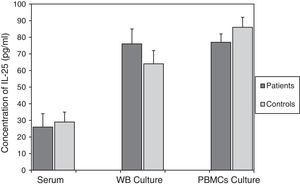
According to the results in Fig. 2, there were no significant differences between IL-25 levels in serum, mitogen-activated WB culture supernatants and mitogen-activated PBMC culture supernatants in the asthmatics (26±6pg/ml, 76±8pg/ml and 77±6pg/ml, respectively) compared to the corresponding values obtained for the control group (29±8pg/ml; p=0.773, 64±9pg/ml; p=0.348 and 86±5pg/ml; p=0.298, respectively).
Comparison of IL-25 levels in samples from the patients with asthma and the non-asthmatic control group. There were no statistically significant differences between serum IL-25 levels (p=0.773), or IL-25 levels in the supernatants of WB (p=0.348) and PBMC (p=0.298) cultures activated by mitogens PHA (5μg/ml) and LPS (25μg/ml), in the asthmatic patients and controls. Data are presented as mean±SEM.
Although there was no difference between the differential WBC counts of the two study groups, the total WBC count of patients with severe asthma was increased in comparison with total WBC count of the healthy controls. It is well documented that in allergic asthma eosinophil recruitment to the airway will be increased.13,24,25 However, the eosinophil counts in the blood of severe asthmatic patients were not different from the eosinophil counts in the blood of the healthy controls.
In this study, we could not demonstrate any increase in the levels of IL-22 or IL-25 in the serum, in the supernatant of mitogen-activated WB culture nor in the supernatant of mitogen-activated PBMC cultures of the patients with severe asthma in comparison with the healthy controls.
These findings are in partial disagreement with study of Zhao et al., in that the plasma concentration of IL-22 in nine severe asthmatic patients (three men, six women) was significantly increased in comparison to the healthy controls.16 In addition, in another study by Farfariello et al., they revealed that expression of IL-22 mRNA in unstimulated PBMCs of nine asthmatic children, only three of them with history of severe asthma, tend to increase with the severity of asthma and were increased in the patients with severe asthma.15
It is well documented that IL-22 levels will increase in the lungs of patients with asthma.13,26 This seems to be required for the onset of allergic airway inflammation, but has an inhibitory function during the effector phase.26 One possible explanation for the different IL-22 levels between the lung and serum of asthmatic patients in comparison with healthy people is that the IL-22-producing leukocytes are strongly enriched in the lung as compared to the circulation and their function should be considered in the context of the target organ.27
Our results are consistent with the work of Zhao et al., which provided evidence that the plasma concentration of IL-25 tended to decrease with severity of asthma and was not different in the serum of patients with severe asthma. In that study, the levels of IL-25 in the culture supernatants of activated PBMCs from asthmatics and healthy controls were similar.16 According to other studies that found increases in the level of IL-25 during asthma it can be concluded that IL-25 levels in serum and in the airways will be suppressed due to the effect of some other factors in chronic severe asthma. However, further experiments are needed to assess the concentration of IL-22 and IL-25 in the blood to give accurate information about their systemic functions in the pathogenesis of asthma. It can be concluded that IL-22 and IL-25 levels in the blood of patients with severe asthma are not higher than normal persons, and their roles in the immunological processes involved with asthma are related to their local actions.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in the study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients mentioned in the article. The author for correspondence is in possession of this document.
Conflict of interestThis project (approved no: p/16/35/153747) was funded by the Research Deputy of Hamadan University of Medical Sciences, Hamadan, Iran. The authors declare they have no conflict of interests.
We would like to express our gratitude to Dr. Brett Baillie (Australian Institute of Marine Science, Australia) for reading and editing the manuscript. The authors also wish to thank the staff of Shahid Beheshti Hospital and Research Center for Molecular Medicine for their help in carrying out this project.