Cow's milk protein allergy (CMPA) represents one of the leading causes of food allergy in infants and young children. The immune reaction may be IgE mediated, non-IgE mediated, or mixed. IgE-mediated cow's milk protein allergy is revealed by immediate and acute symptoms which can be severe. The aim of this study is to report a one centre experience in the real life of testing children with IgE-mediated CMPA and try to identify predictive factor for follow-up challenges.
MethodRetrospective and monocentric study between September 1997 and February 2008. 178 infants diagnosed with IgE-mediated CMPA during breastfeeding weaning were included. Initial factors such as age, sex, skin prick tests (SPTs), specific IgE (sIgE), atopic dermatitis and types of reaction were noted. Between 12 and 24 months all infants have undergone at least one evaluation including SPT.
ResultsAt the food challenge, 138 (75.8%) infants were found tolerant. Results of the skin prick test (SPT) were statistically different according to the food challenge result (2.2mm vs. 5.1mm, p<0.0001). It was the same result for sIgE for CM 2.0ku/l vs. 11.5ku/l – p<0.0001 and for casein 1.0ku/l vs. 16.0ku/l – p=0.0014.
ConclusionThis study confirms the practical interest of both SPT and sIgE in the evaluation of tolerance induction in IgE-mediated CMPA, but with no corresponding results. Sensitivity, specificity and probability curves of success for cow's milk challenge can be determined and have clinical utility.
Cow's milk protein allergy (CMPA) represents one of the leading causes of food allergy in infants and young children.1 The immune reaction may be IgE mediated, non-IgE mediated, or mixed.2 IgE-mediated cow's milk protein allergy is revealed by immediate and acute symptoms which can be severe (e.g. anaphylaxis). These symptoms, in most cases, are associated with positive skin prick test and/or serum specific IgE (sIgE). In a clinical approach, these data confirm the diagnosis of IgE CMPA and are considered as sufficient arguments to introduce cow's milk protein (CMP) eviction. CMP Challenge (CMPC) is rarely performed in our institution, to confirm the diagnosis, but rather after six months of elimination diet.2 An oral food challenge is certainly the “gold standard” for establishing the diagnosis of food allergy, but CMPA evolution is usually favourable and patients should be re-evaluated every 6–12 months to assess whether they have developed tolerance to CMP, and CMPC is usually proposed before reintroduction between 12 and 24 months of age.2 The clinician has to establish when it seems reasonable to try food challenge and to reintroduce CMP and which criteria are useful: does he have to wait for the clearing of SIgE or SPT? Does the success depend on the initial seriousness of allergy? Do reliable predictive factors exist to avoid a premature or even dangerous food challenge?
The aim of this study is to report our real life centre experience in a selected group of IgE-mediated CMPA patients and to try to identify predictive criteria of the CMPC result.
Materials and methodsPatients and monitoringThis retrospective clinical study was conducted from September 1997 to February 2008 in the allergology unit (Department of Pediatric Gastroenterology, Hepatology and Nutrition, Children's Hospital of Lyon). One hundred and seventy-eight children who have presented an immediate-type reaction after the ingestion of cow's milk protein (CMP) during breastfeeding weaning were included. Initial diagnosis of the CMPA was based on clinical data including: familial history, age, sex, eczema, milk consumption history and evocative symptoms (severity rating 1–4) with a reaction to CMP ingestion within an hour. Reactions were classified in four levels of severity. Level 1: urticaria or local angio-oedema (e.g. local facial reactivity); level 2: generalised urticaria; level 3: two organs involved (e.g. lung and skin); level 4: serious symptoms (three organs) and/or anaphylaxis (evidence of hypotension). After the initial reaction, some children had the diagnosis of cow's milk allergy confirmed with a prick test and detection of IgE specific.
Throughout the study, the same paediatric allergist was in charge of the monitoring: (1) he decided if the diagnosis of IgE-mediated CMPA can be retained and initiate the CMP avoidance; (2) he performed clinical monitoring of sIgE and/or SPT at 12 months, then every six months and at least two months prior to the food challenge; and (3) he decided the time of the CMPC, according to clinical evaluation, sIgE and/or SPT.
Skin prick testsSPT were performed using Stallerpoint® (Stallergènes SA laboratories). If the child had anti-histamine treatment for any reason, this treatment is stopped one week before the skin testing. Three solutions were used for this test: UHT semi-skimmed cow's milk for the allergen (cow milk standardised commercial extracts did not exist in France), a solution of histamine hydrochloride 1% (or codeine phosphate) for the positive control, and saline solution for the negative control. The readings of the test were taken 20min after. The SPT were considered positive if the diameter of induration was 3mm more than the negative control.
Immunological testsThe IgE dosages against cow's milk, casein, α-lactalbumin and β-lactoglobulin were performed according to the CAP System® technique (Pharmacia) following the manufacturer's instructions. The values were expressed in ku/l; those inferior to 0.35ku/l (and since 2007, inferior to 0.10ku/l, year of the introduction of this value in France) were considered as undetectable.
Basophil activation tests could have been very interesting in the evaluation of the food specific IgE-mediated reactions but they cannot be used in a routine process in France.
Evaluation of clinical reactivityAll the children had at least one food challenge. The food challenge was done during a one-day hospitalisation, under medical surveillance and with parental agreement. The protocol consisted in giving each child UHT semi-skimmed cow's milk, in open, in quantities of 1 drop, 3 drops, 10 drops, 2millilitres (ml), 5ml, 20ml and then 50ml, every 20–30min interval; that is 80ml in 3h. The child was under observation during at least 2h in the unit after the last cow's milk intake.
The food challenge was negative when there was no reaction. In this case cow's milk is fully and progressively re-introduced into the child's diet. CMPC was positive when we noticed a reaction to CMP ingestion within an hour. In this case, the elimination diet was continued for 6–12 months.
Statistical analysisThe different studied factors have been compared with the result of the food challenge. Categorical variables were compared using a Chi2-test or a Fisher's exact test. The quantitative variables were analysed using a Wilcoxon Mann–Whitney test or a Student test after checking whether or not hypothesis of Normal distribution was confirmed (Kolmogorov–Smirnov tests). Sensitivity and specificity were calculated for the skin prick test and the immunological test for cow's milk as well as for the final check-up. For all the statistical tests used, differences between variables were considered as statistically significant for a p-value<0.05. The ROC (Receiver Operating Characteristic) curve that presents the relation between sensitivity and specificity of a test curve was established for specific IgE tests, using sensitivity values and 1 – specificity. Univariate and age and sex adjusted logistic regression was used to evaluate how increasing level of casein and CM sIgE and induration size were associated with the risk of success.
Fitted predicted probability curves with bounding 95% confidence limit were also calculated, using results from the univariate logistic regression for casein and CM sIgE rates and the diameter of SPT wheal in order to assess the probability of success of the food challenge and so allow us to predict tolerance.
Statistical analyses were conducted using SAS version 9.1.3 (SAS Institute Inc., NC, USA).
ResultsOne hundred and seventy-eight children with a sIgE-mediated immediate allergic reaction after the intake of cow's milk during breastfeeding withdrawal participated in this homogeneous cohort (Table 1). The study population was composed of 89 girls and 89 boys, with a mean age at initial reaction of 3.67 months (SD: 2.4) and a mean age during food challenge of 17.63 months (SD: 10.1). The main symptom of initial reaction was generalised urticaria (47.8%) and there were serious symptoms (grade 4) in four patients (2.2%). We noticed that 33.1% of children have atopic dermatitis.
Clinical characteristics of the 178 infants.
| Variables | N (%) |
| Gender | |
| Girls | 89 (50.0) |
| Boys | 89 (50.0) |
| Atopic dermatitis | 58 (33.1) |
| Missing | 3 |
| Type of initial reaction | |
| - Level 1: localised oedema or urticaria | 44 (25.3) |
| - Level 2: generalised urticaria | 83 (47.7) |
| - Level 3: 2 organs involved | 44 (25.3) |
| - Level 4: 3 organs involved and/or anaphylaxis | 47 (27.0) |
| Missing | 4 |
| Before the milk challenge | |
| - Positive for cow's milk IgE | 97 (63.0) |
| Missing | 25 |
| - Positive for casein IgE | 53 (44.9) |
| Missing | 60 |
| - Positive for SPTb(mm) | 66 (44.6) |
| Missing | 30 |
| Age at first reaction (months) | 3.67 (2.4)a |
| Age at the milk challenge (months) | 17.63 (10.1)a |
| SPT (before challenge, mm) | 2.82 (3.2)a |
| Missing | 30 |
| Cow's milk sIgE (before challenge, ku/l) | 4.31 (23.6)a |
| Missing | 24 |
| Casein sIgE (before challenge, ku/l) | 4.56 (30.6)a |
| Missing | 60 |
In 75.8% of the cases (n=135) the food challenge was negative with no reaction at home after consuming larger amounts of milk. The comparison of initial factors and final factors according to the food challenge result is presented in Table 2. The presence of atopic dermatitis and the type of initial clinical reaction did not have a significant influence on the result. There were serious symptoms in four patients (2.2%) but no patient with positive reaction was treated with epinephrine due to hypotension. There was no statistical difference between mean age at food challenge according to the food challenge result (p=0.10).
Comparison between initial and final parameters according to milk challenge results.
| Factors | Success CMPC | Failure CMPC | p-Value |
| n=135 (%) | n=43 (%) | ||
| Gender | |||
| Girls | 68 (50.4) | 21 (48.8) | 0.861a |
| Boys | 67 (49.6) | 22 (51.2) | |
| Atopic dermatitis | |||
| No | 93 (70.4) | 24 (55.8) | 0.077a |
| Yes | 39 (29.6) | 19 (44.2) | |
| Missing | 3 | 0 | |
| Type of initial reaction: | |||
| Level 1: localised oedema or urticaria | |||
| No | 97 (74.0) | 33 (76.7) | 0.724a |
| Yes | 34 (26.0) | 10 (23.3) | |
| Missing | 4 | 0 | 0.595a |
| Level 2: generalised urticaria | |||
| No | 67 (51.2) | 24 (55.8) | 0.959a |
| Yes | 64 (48.9) | 19 (44.2) | |
| Missing | 4 | 0 | 0.345a |
| Level 3: 2 organs involved | |||
| No | 98 (74.8) | 32 (74.4) | |
| Yes | 33 (25.2) | 11 (25.6) | |
| Missing | 4 | 0 | |
| Level 4: 3 organs involved and/or anaphylaxis | |||
| No | 98 (74.8) | 29 (67.4) | |
| Yes | 33 (25.2) | 14 (32.6) | |
| Missing | 4 | 0 | |
| Age at initial reaction (months) | 3.52 (2.42) | 4.12 (2.28) | 0.06b |
| Missing | 1 | 1 | |
| Age at the milk challenge (months) | 17.3 (10.7) | 18.6 (7.5) | 0.10b |
| Missing | 4 | 1 | |
| Cow's milk sIgE (before milk challenge, ku/l) | 2.0 (6.5) | 11.5 (45.9) | |
| Missing | 19 | 5 | <0.0001b |
| Casein sIgE (before the milk challenge, ku/l) | 1.0 (3.6) | 16.0 (62.0) | 0.0014b |
| Missing | 45 | 15 | |
| Induration size (before the milk challenge, mm) | 2.2 (2.8) | 5.1 (3.5) | <0.0001b |
| Missing | 21 | 9 | |
We observed that the mean result of the SPT was statistically different according to the food challenge result (2.2mm vs. 5.1mm, p<0.0001). It was the same for the mean result of immunological tests (CM sIgE 2.0ku/l vs. 11.5ku/l – p<0.0001 and sIgE casein 1.0ku/l vs. 16.0ku/l – p=0.0014, respectively).
Comparison of SPT and sIgE showed no corresponding results for SPT and sIgE as either positive or negative (Table 3).
Absolute numbers and percentages for different combinations of positive and negative specific IgE (sIgE) and skin prick test (SPT) before the oral food challenge test.
| Cow milk sIgE | ||
| Negative | Positive | |
| SPTa | ||
| Negative | 40 (29.9) | 33 (24.6) |
| Positive | 7 (5.2) | 54 (40.3) |
| Casein sIgE | ||
| Negative | Positive | |
| SPTa | ||
| Negative | 42 (43.3) | 13 (13.4) |
| Positive | 9 (9.3) | 33 (34.0) |
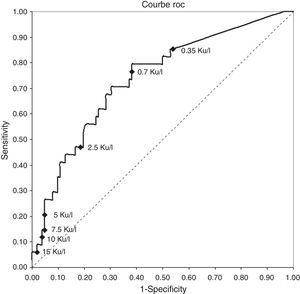
The ROC curve for cow's milk sIgE rates in children of younger than two at the time of food challenge (most relevant group n=153) was calculated for all possible threshold values (Fig. 1). This curve indicates to the clinician that the immunological test with a threshold value of 5ku/l induces almost 5% of negative challenge (success CMPC) and a test with a threshold value of 0.35ku/l almost 15% positive challenge (failure CMPC). The area under the curve which characterises the global quality of the test is 71% (standard error=0.0475; p<0.0001).
The Predictive Positive Value (PPV) and Predictive Negative Value (PNV) of different diameters of SPT and levels of cow's milk sIgE to predict the results of the food challenge are shown in Table 4. Increasing size of the induration from 1 to 10mm increases the NPV but the difference between 1 and 10mm did not largely improve the capacity of prediction of failure to CPMC.
Positive and negative predictive value of success for Cow's milk specific IgE level and skin prick test (SPT) induration diameter.
| PPV | NPV | |
| Level of cow's milk specific IgE | ||
| 0.30ku/l | 0.89 | 0.32 |
| 0.75ku/l | 0.87 | 0.37 |
| 2.0ku/l | 0.83 | 0.43 |
| 3.0ku/l | 0.80 | 0.50 |
| 4.0ku/l | 0.78 | 0.50 |
| 5.6ku/l | 0.77 | 0.44 |
| 6.5ku/l | 0.77 | 0.50 |
| 7.1ku/l | 0.77 | 0.60 |
| Diameter of SPT induration diameter | ||
| 1mm | 0.71 | 0.79 |
| 2mm | 0.70 | 0.80 |
| 3mm | 0.53 | 0.80 |
| 4mm | 0.52 | 0.83 |
| 5mm | 0.53 | 0.85 |
| 6mm | 0.44 | 0.86 |
| 7mm | 0.37 | 0.85 |
| 8mm | 0.36 | 0.88 |
| 9mm | 0.35 | 0.91 |
| 10mm | 0.34 | 0.92 |
Increasing the level of cow's milk IgE from 0.35 to 7ku/l, doubled the PNV and did not significantly decrease the PPV.
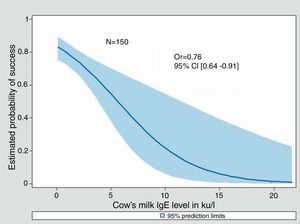
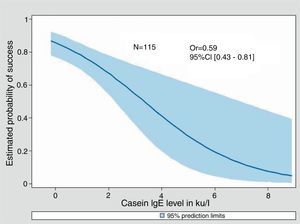
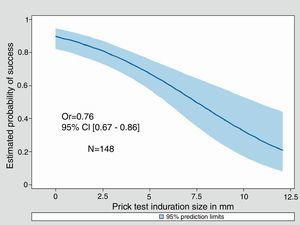
Univariate Odds Ratios were calculated for cow's milk sIgE, Casein sIgE and induration size. For all three, an increase was significantly associated with a decreasing probability of success in the milk challenge (0.76 [0.64–0.91], 0.59 [0.43–0.81] and 0.76 [0.67–0.86] respectively). Adjustment for sex and age at milk challenge did not change the parameters value (data not shown). Only 89 out of 175 children underwent the three tests.
We were unable to determine in this study a diameter of SPT or a value of sIgE (for cow's milk or casein) predicting 90–95% of positive challenges.
Fitted predicted probability curves of success of the food challenge were created for cow's milk sIgE, casein sIgE, and the diameter of induration of the skin prick test (Figs. 2–4). The corresponding cow's milk sIgE level giving an 80% chance of success was 0.85ku/l. It was 0.73ku/l for casein sIgE level and 2mm of induration (i.e. negative SPT) for the same probability of success.
CMA is more often transitional and tends to rapidly decrease in the first two years of life: 51% recovery at 2 years and to decrease gradually afterwards: 81% recovery at 5 years and 89% recovery at 8½ years.3 Essentially, if CMA is common, it is not always easy to determine when tolerance has been established and when it is safe to undertake challenge and liberalise the diet. This retrospective observational study presents several biases, but we believe that this experience is interesting because it corresponds to what is observed in real life and not in selected patients.
First, we can notice that in 75.8% of cases (n=138) and at a mean age of 17.7 months CMPC was negative (Table 1). So in our population, the average foreclosure of milk proteins is 14 months and is consistent with the new recommendations of CMP-free diet for a period of at least one year before an oral food challenge.4 Unlike what was usually reported,3,4 we must notice that the type of initial reaction or the presence of atopic dermatitis has no effect on the food challenge result.
This cohort of sIgE-mediated CMPA patients allows us to try to determine positive and negative predictive values for deliberate challenge which are of interest to guide the clinician in his decision to try a food challenge. The decision to undertake challenge was subjective because it was undertaken by the allergist knowing the results of the tests and after discussion with the parents. So, not surprisingly, the results of SPT and sIgE showed much lower results in patients who were able to tolerate the food challenge than in those who did not. The likelihood of not reacting to cow's milk challenge was 80% when the SPT wheal was negative, when cow's milk sIgE was 0.85ku/l and 0.73ku/l for casein specific IgE, which are also low levels.
Many factors, related to the food challenge outcome, have been highlighted in a birth prospective cohort of 6209 children,3 showing that urticaria and atopic dermatitis are predictive symptoms of a failure in food challenge (p-value<0.001 and 0.05 respectively). The SPT has a good sensitivity, ranging from 61% to 83%, but with a less important specificity, ranging from 51% to 72%.5–7 A skin prick test with a diameter≤3mm would have a negative predictive value of 98%, and a test ≥15mm a positive predictive value of 95%,7 but the predictability of this test is not sufficient to substitute the food challenge. Other studies6,8 underlined that cow's milk specific IgE (CM sIgE) concentration in serum, is correlated with the result of reintroduction tests. Two studies9,10 were mainly interested in rates of sIgE in casein, β-lactoglobulin and α-lactalbumin. They revealed non-statistical differences between groups of children who have passed their food challenge or those who have failed. For clinical practice the determination of sIgE and SPT are useful diagnostic tests in children, but a combination of the two tests is not recommended for the diagnostic.11 Mehl et al.12 have recently reported that 23% (of 395 children) of orally challenged patients with cow's milk showed no corresponding results for SPT and sIgE as either positive or negative. In this work, we also report comparable numbers (Table 3).
This retrospective observational study also presents selection bias. There was only one physician deciding during all the study but his decision criteria can change with his experience during the long time of the study. Food challenges were performed according to implicit criteria (e.g. check-up tests results and child age) and also when he thought he had chances of success. This clearly affected the probability to react during the food challenge, which is lower than in prospective randomised studies but it is also a reflection of the real life.
The ROC curve for cow's milk sIgE rates in children of less than two at the food challenge (most relevant group n=153) (Fig. 1) is a visual tool to rapidly assess the performance of multiple threshold value test.
The probabilities of success curves are useful to visualise the chance of success of the food challenge and help the clinician to take his decision. So a cow's milk sIgE at 5ku/l is associated to a 55% chance of success, and a rate at 2.5ku/l as well as a skin test of 5mm is associated to at least 70% chance of success of the food challenge. These probability curves have been used by Östblöm et al.13 for several allergens, on a population of 197 children of four years of age, with the same interest for decision-making aids and almost the same probabilities for the cow's milk sIgE. Moreover, in a recent publication, Zomer-Kooijker et al.14 using pre-challenge standardised questionnaires, skin prick tests (SPTs), and specific IgE levels (sIgE), developed a risk score to predict the CMPC issue in patients with CMPA and other food allergy.
ConclusionThis study, is retrospective and monocentric, but is in the real life clinical practice.
We confirm that the results of SPT and sIgE seem promising as a complementary tool to predict the food challenge outcome. We also suggest predicted probability curves, according to cow's milk sIgE and casein sIgE rate and the diameter of SPT as an additional decision tool for clinicians. Their ability to quantify the probability of success of cow's milk challenge needs validation in a prospective study.
Ethical disclosuresProtection of human subjects and animals in researchProtection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this investigation.
Patients’ data protectionConfidentiality of data. The authors declare that no patient data appear in this article.
Right to privacy and informed consentRight to privacy and informed consent. The authors declare that no patient data appear in this article.
Conflict of interestThe author declares no conflict of interest
The authors would like to thank Claudine Vaudo, master student, for her help in collecting data, Pr Anne-Marie Schott-Pethelaz for her reviewing time and Neal Kent for reviewing the english wording.