Atopic dermatitis is a chronic, relapsing, highly pruritic, inflammatory skin disease characterized by typical localization with increasing prevalence of 10–20% in children. Pruritus is one of the major diagnostic criteria of atopic dermatitis and also the main complaint altering quality-of-life of affected patients, inducing and aggravating inflammation. Although pruritus is the absolute symptom of AD, the etiology has not been fully explained yet and current antihistamine therapies are ineffective.
The aim of the study was to assess the correlation between IL-31 level and disease severity in patients with atopic dermatitis through Severity SCORing of Atopic Dermatitis (SCORAD) index and the degree of itching assessed subjectively.
Material and methodsOne hundred thirty-five children were enrolled in the study in total, 70 children with diagnosis of atopic dermatitis and 65 healthy children in control group. Data on demographic features (age, gender, family history of atopy) and laboratory values of serum eosinophil, total IgE, IgM, IgA, IgG levels and skin prick test results were collected through patient files. The disease severity was assessed by SCORAD index. IL-31 levels were measured with human IL-31 ELISA kit.
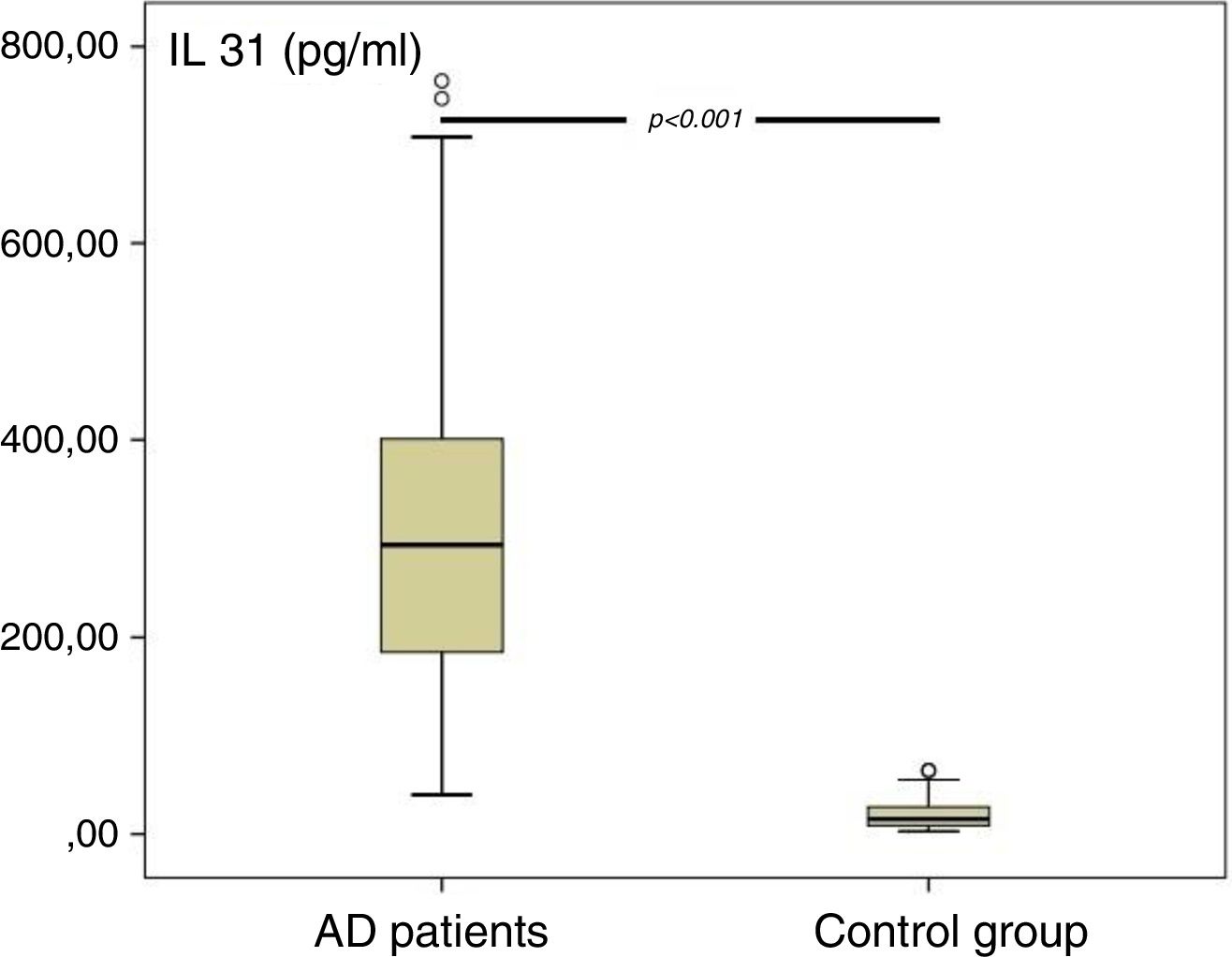
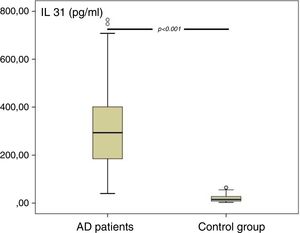
ResultsThe statistical analysis showed that IL-31 level was significantly higher in AD patients than in the control group (AD vs CG, p 0.0001). There was no significant difference in IL-31 levels between the three subgroups divided according to the SCORAD severity score (p:0.27).
ConclusionIL-31 levels were significantly higher in AD patients compared to control group but irrelevant to the disease severity.
Atopic dermatitis is a chronic, relapsing, highly pruritic, inflammatory skin disease characterized by typical localization with increasing prevalence of 10–20% in children.1 Pruritus is one of the major diagnostic criteria of atopic dermatitis and is also the main complaint altering quality-of-life of affected patients, inducing and aggravating inflammation.2 Although pruritus is the absolute symptom of AD, the etiology has not been fully explained yet and current antihistamine therapies are ineffective. However, the role of several mediators other than histamine such as neuropeptides, neurotransmitters, cytokines, proteinases and arachidonic derivates were revealed in recent studies suggesting new therapeutic approaches.3 Among them, IL-31 is a newly discovered cytokine associated with chronic skin inflammation and pruritus in allergic diseases.4 It is related to the IL-6 cytokine family, expressed mainly by activated CD4+ T cells and signals through a heterodimeric receptor consisting of IL-31 receptor alpha(IL-31RA) and oncostatin M receptor beta (OSMR) which are expressed on keratinocytes, monocytes and dorsal root ganglia, producing late onset itch.5–8 Over-expression of IL-31 in mice models induced severe pruritus and chronic dermatitis.5 In patients with atopic dermatitis, not only activated leukocytes expressed significantly higher IL-31 levels, but also protein expression of IL-31 receptor components was increased.6,9 Moreover, IL-31 is associated with impaired barrier function through basal cell proliferation in the epidermis.10 Various studies assessed the link between IL-31 level and the severity of the disease and found controversial data.
The aim of the study was to assess the correlation between IL-31 level and the severity of the disease in patients with atopic dermatitis through Severity SCORing of Atopic Dermatitis (SCORAD) index and the degree of itching assessed subjectively.
MethodsWe consecutively included patients with AD who were followed at Istanbul University Istanbul Faculty of Medicine, Pediatric Allergy and Immunology outpatient clinic between January and June 2013. One hundred thirty-five children were enrolled in the study in total, 70 children with diagnosis of atopic dermatitis (29 female, 41 male) and 65 healthy children in control group. The diagnosis of atopic dermatitis was made according to Hanifin-Rajka criteria by a physician. Data on demographic features (age, gender, family history of atopy) and laboratory values of serum eosinophil, total IgE, IgM, IgA, IgG levels and skin prick test results were collected through patient files. Our set of skin prick tests included the aeroallergens (Dermatophagoides pteronyssinus, Dermatophagoides farinae, Rumex acetosa, Urtica dioica, Plantago, Artemisia vulgaris, Chenopodium album, Parietaria officinalis, Lolium perenne, Anthoxanthum odoratum, Dactylis glomerata, Festuca elatior, mixture of seven cereals (barley, maize, oat, rice, rye, wheat, wheat flour), Alnus glutinosa, Fagus sylvatica, Betula alba, Corylus avellana, Maple, Quercus robur, Olea europaea, Populus alba, Salix caprea, False acacia, Pinus sylvestris, cat and dog epithelia, latex, Cladosporium, Aspergillus and Alternaria) and food allergens (milk, egg white, egg yolk). The severity of the disease was assessed by SCORAD index, which also included subjective itch intensity. Subjective itch intensity was measured with a patient/parent-reported 0–10 scale (0: no pruritus, 10: extremely severe pruritus).
Blood collectionVenous blood samples from all patients and healthy controls were collected by antecubital veni puncture directly into K3-EDTA tubes. Plasma separation was performed after centrifugation of EDTA whole blood samples at 1500×g for 10min. Plasma specimens were kept frozen at −80°C until analysis.
Biochemical analysisIL-31 levels were measured with human IL-31 ELISA kit (Diaclone, France) using Triturus analyzer (Grifols International, Spain) according to the manufacturer's instructions.
Statistical analysisA statistical analysis was performed using IBM SPSS 22 (IBM, NY, USA). A Shapiro–Wilk test was used to test distributions for normality. Parametric data were expressed as the mean±standard deviation (SD), and non-parametric data were expressed as the median, inter-quartile range (IQR). The Kruskal Wallis test was used to compare more than two independent parameters and a Mann–Whitney U test was used to compare medians between groups. Correlation between two variables was assessed using the Spearman rank correlation coefficient. Categorical data were evaluated using the chi square test and p<0.05 was accepted as statistically significant.
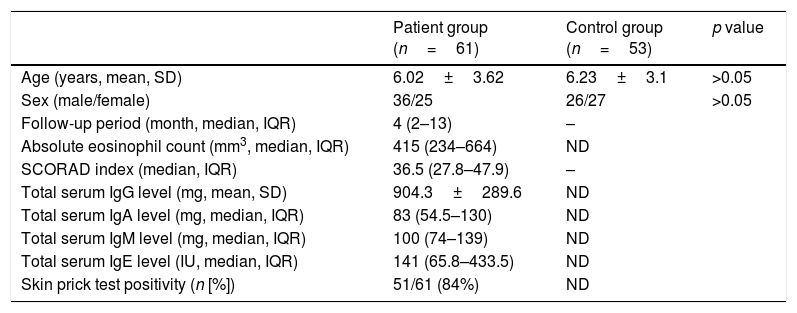
ResultsTable 1 shows clinical and laboratory features of the patients. Mean age of the study and the control group was similar (six years).
Demographic and clinical features of the study and control groups.
| Patient group (n=61) | Control group (n=53) | p value | |
|---|---|---|---|
| Age (years, mean, SD) | 6.02±3.62 | 6.23±3.1 | >0.05 |
| Sex (male/female) | 36/25 | 26/27 | >0.05 |
| Follow-up period (month, median, IQR) | 4 (2–13) | – | |
| Absolute eosinophil count (mm3, median, IQR) | 415 (234–664) | ND | |
| SCORAD index (median, IQR) | 36.5 (27.8–47.9) | – | |
| Total serum IgG level (mg, mean, SD) | 904.3±289.6 | ND | |
| Total serum IgA level (mg, median, IQR) | 83 (54.5–130) | ND | |
| Total serum IgM level (mg, median, IQR) | 100 (74–139) | ND | |
| Total serum IgE level (IU, median, IQR) | 141 (65.8–433.5) | ND | |
| Skin prick test positivity (n [%]) | 51/61 (84%) | ND |
IQR, interquartile range; SD, standard deviation; ND, not determined, Ig, immunoglobulin.
A significant difference was found in IL-31 levels of AD patients and control group (Fig. 1). The statistical analysis showed that IL-31 level was significantly higher in AD patients than in the control group (AD vs. CG, p 0.0001).
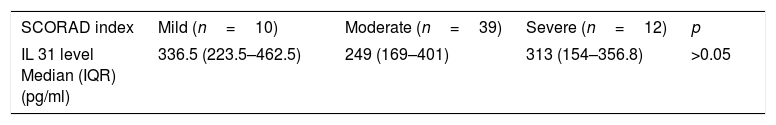
We categorized the AD group into mild, moderate, and severe AD groups, based on the SCORAD index. Among them, 39 patients were moderate and 12 patients were severe. There was no significant difference in IL-31 levels between the three subgroups divided according to the SCORAD severity score (p:0.27) (Table 2).
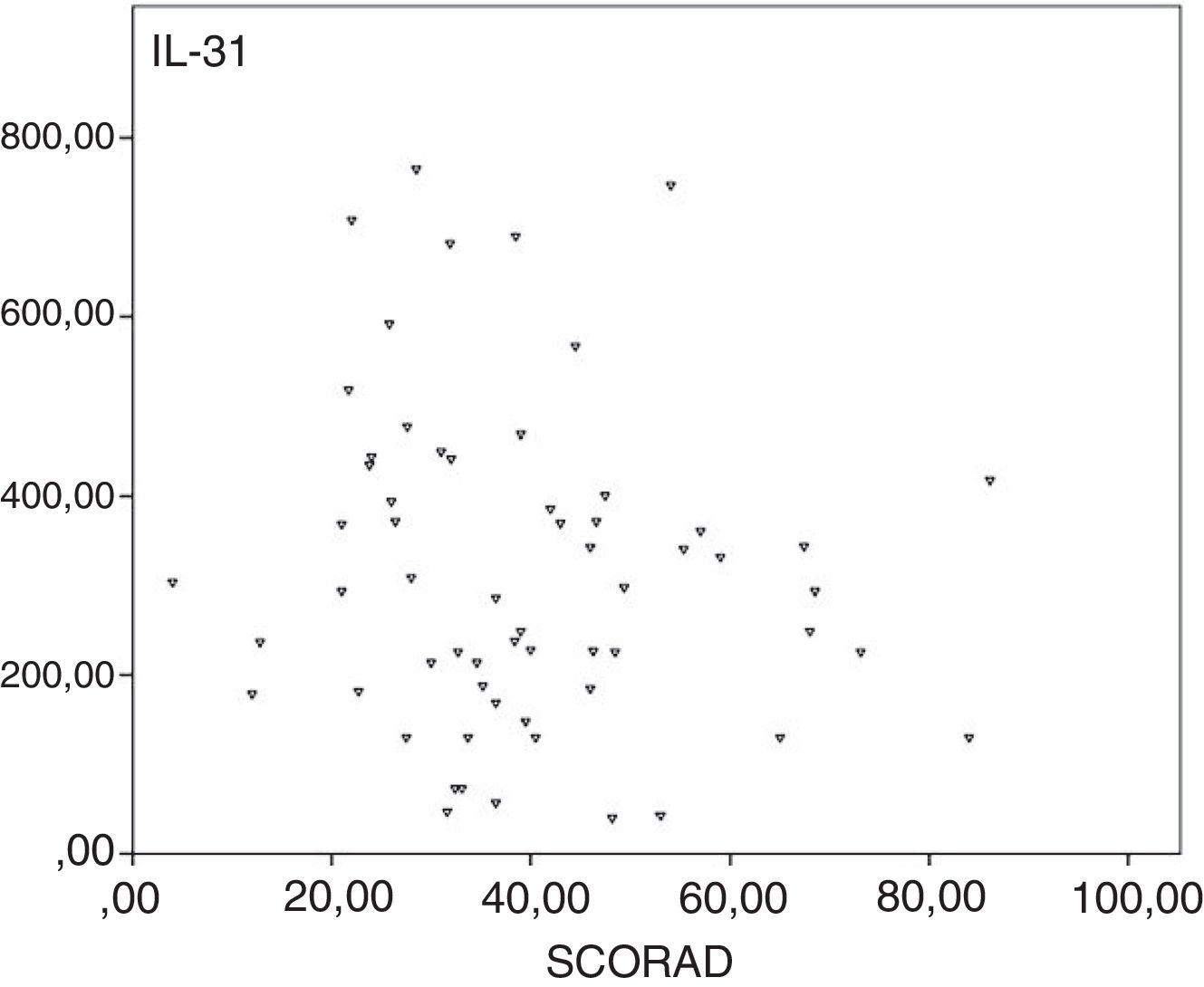
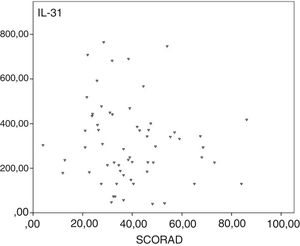
We assessed IL-31 levels of the control group and the SCORAD subgroups. We found the highest difference between the control group and the mild SCORAD group. Correlation analysis did not show any statistical significance between SCORAD and plasma IL-31 levels (rho=−0.154, p>0.05; Fig. 2).
Plasma IL-31 level was not related with age, gender, serum total IgE, IgG, IgM, IgA levels, serum eosinophil counts and skin prick test positivity nor with SCORAD index and subjective pruritus score, significantly (p>0.05).
We also classified the patients as atopic and non-atopic according to serum IgE levels and skin prick tests. Patients with serum total IgE≥100ku/L and/or skin prick test positivity to one or more allergens were considered as atopic. Majority of the patients (n=53, 83%) were atopic. No significant relation was found between serum IL-31 levels and atopic status (p:0.814).
DiscussionWe aimed to detect whether IL-31 level is increased in AD patients and if it is related to disease severity. In the present study, IL-31 level was significantly higher in AD patients compared to the control group but irrelevant to disease severity.
The relationship between IL-31 level and the disease severity in patients with atopic dermatitis has been assessed in various studies. Some studies have suggested a correlation between serum IL-31 levels and the disease severity, but others have not.11–15 Kim et al. checked the laboratory values, severity score, and serum IL-31 levels in 55 AD patients, 34 with allergic type AD, 21 with non-allergic type AD and 38 healthy, non-atopic controls. They also measured IL-31 mRNA levels in lesional skin in 13 subjects with AD and in four controls. They found significantly higher levels of serum IL-31 that were associated with serum IgE, disease severity, and subjective itch intensity in AD patients and their IL-31 mRNA levels from the lesional skin samples also correlated with serum IL-31 level.11 Raap et al. analyzed IL-31 serum levels together with IL-4, IL-13, ECP, total IgE levels and SCORAD score, sleeplessness, and pruritus severity in 60 children with extrinsic, in five children with intrinsic atopic dermatitis, and 20 non-atopic healthy children. IL-31 serum levels significantly correlated with SCORAD score (p<0.01), sleeplessness (p<0.05), IL-4, and IL-13 levels (p<0.01) in children with extrinsic atopic dermatitis but they found no correlation of IL-31 with pruritus, total IgE, and ECP levels.13
In contrast, Siniewicz-Luzeńczyk et al. measured serum IL-31 level and assessed the disease severity in 25 children with AD and found no statistical correlation between serum IL-31 level and itch intensity and disease severity.14 Furthermore Neiss et al. found increased mRNA levels of IL-31 in biopsy specimens taken from patients with AD, irrespective of the severity of the disease and serum IgE levels.16
We found no significant correlation between IL-31 levels and disease severity (SCORAD index and subjective pruritus score) in AD patients. This may be due to subjective pruritus assessment in the child population. IL-31 levels were also not significantly related with age, gender, serum total IgE, IgG, IgM, IgA levels, serum eosinophil counts and skin prick test positivity in our study. Measuring serum IL-31 level may not be as accurate as measuring IL-31 level in skin lesions. More studies on a larger population may be conducted to evaluate IL-31 levels in skin biopsies during acute and remission phases in AD patients.
ConclusionIL-31 levels were significantly higher in AD patients compared to the control group but irrelevant to disease severity.
Conflict of interestThe authors have no conflict of interest to declare.