Epidemiological evidence suggests the existence of a direct link between allergic rhinitis (AR) and asthma. Several studies also support the presence of small-airway dysfunction (SAD) in non-asthmatic children with AR. However, it remains unknown whether SAD can predict the progression of AR to asthma. Our objective was to explore the existence of SAD in non-asthmatic children with AR and to assessed its ability to predict the development of asthma.
MethodsSeventy-three 6-year-old children with intermittent moderate-severe AR but without asthma symptoms/medication within the last two years, underwent spirometry and measurement of respiratory resistance (Rrs) and reactance (Xrs) before and after bronchodilation (BD) (300mcg salbutamol). Lung function measurements were performed in the absence of nasal symptoms and repeated at AR exacerbation. SAD was defined as >30% decrease in Rrs or >50% increase in Xrs at 6 or 8Hz post-BD. Participants were followed for five years.
ResultsTwenty-three children (31.5%) developed asthma; this group presented significant post-BD changes in Rrs and Xrs, but only at AR exacerbation. The ability of these changes to predict the development of asthma was exceptional and superior to that of the spirometric parameters. SAD (22 children, 30.1%), emerged as the single most efficient predictor of asthma, independently of other risk factors such as parental asthma, personal history of eczema and type of allergic sensitisation.
ConclusionSAD precedes the development of asthma in children with AR. Changes in respiratory impedance at AR exacerbation may assist in identifying those at risk to progress to asthma.
The concept of “united airways disease”1 supports the existence of a direct link between allergic rhinitis (AR) and asthma. Indeed, epidemiological evidence shows that the two disorders often overlap; the vast majority of patients with asthma experience nasal symptoms, whereas up to 40% of those with AR are also diagnosed with asthma.2 Allergic rhinitis is a recognised risk factor for the development of asthma in both adults3 and children,4 while the severity of nasal symptoms in these patients correlates to the level of asthma control.5,6 Given the above clinical interactions and the well-described pathophysiological mechanisms which support the functional cross-talk between nose and bronchi,2,7 the Allergic Rhinitis and Its Impact on Asthma (ARIA) workgroup proposes that bronchial involvement should be considered in all individuals with AR.8,9
A growing body of evidence suggests that small-airway dysfunction (SAD), as determined by means of subtle spirometric changes, bronchial hyper-reactivity and/or airway inflammation, is an early characteristic of bronchial involvement in non-asthmatic adults with rhinitis.10 Several cross-sectional studies also support the presence of SAD in non-asthmatic children with AR.11–15 However, the evidence on whether SAD is predictive of the development of asthma, remains sparse and controversial16,17; this may be partly attributed to the fact that spirometric parameters cannot reliably detect mild reversible airway obstruction in such patients.14 On the other hand, subclinical changes in respiratory impedance, as determined by the forced oscillations technique (FOT), have been proven superior to spirometry in detecting SAD in non-asthmatic children with AR.14 Nevertheless, no longitudinal study to date has explored the ability of subclinical abnormalities in respiratory impedance to predict the development of asthma in these children.
The aim of the present study was to explore the existence of small-airway involvement, as determined by means of spirometry and FOT, in non-asthmatic school-aged children with AR, and to assess the ability of SAD in predicting subsequent development of asthma.
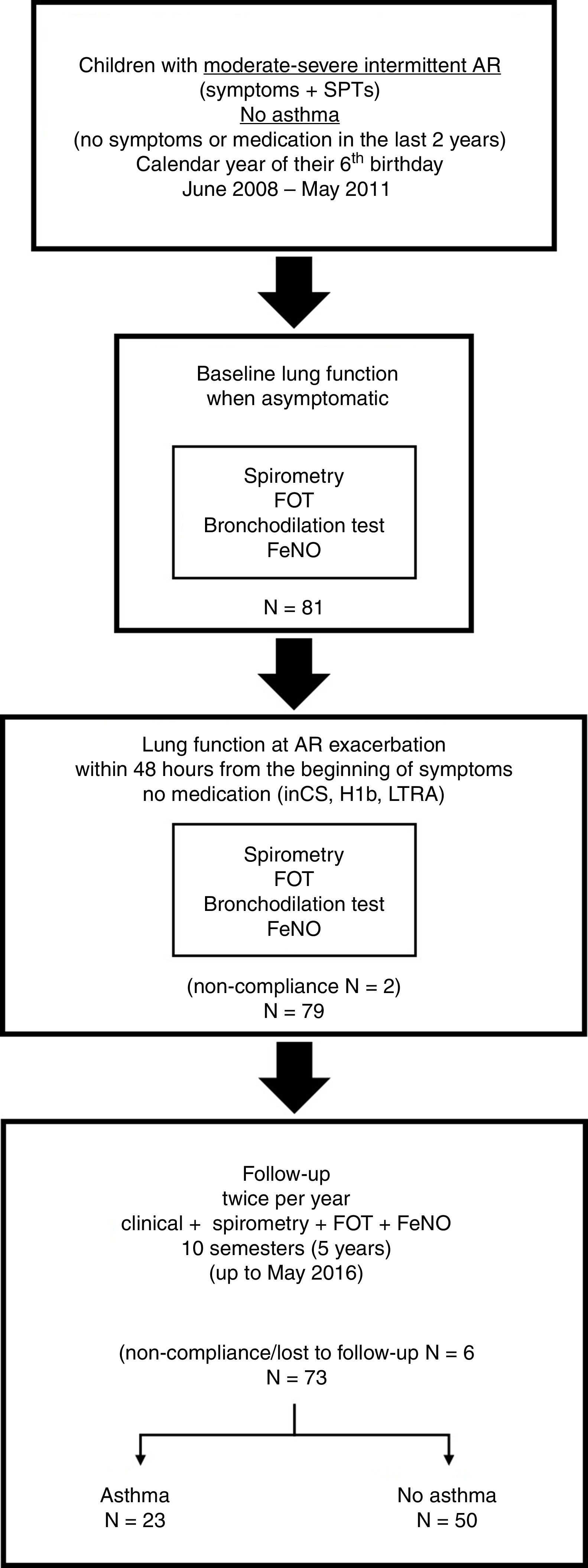
Materials and methodsStudy design and populationThis is a longitudinal study of non-asthmatic children with moderate-severe intermittent AR at the age of six years who were regularly followed for development of asthma up to the age of 11 years. All participants were recruited between June 2008 and May 2011.
Eligible patients were non-asthmatic children cared for in the Paediatric Pulmonology and Allergy Unit of the University Hospital of Patras, Greece, who at the calendar year of their sixth birthday presented symptoms suggestive of AR (i.e., rhinorrhoea, nasal obstruction, nasal itching, and/or sneezing) lasting less than four days per week or less than four consecutive weeks, with troublesome impact on their daily activity (school, sports, social life). Children with mild or persistent forms of AR according to the ARIA classification,8,9 were excluded. Eligible subjects were also required to have positive skin prick tests (SPTs) to common local inhalant allergens (grass pollen mixture, Cynodon dactylon, Olea Europaea, cypress, Parietaria, Dermatophagoides pteronyssinus, Dermatophagoides farinae, Alternaria tensis, Cladosporium herbarum, Aspergillus fumingatus, dog dander, and cat epithelium). SPTs were performed by a paediatric allergy specialist (MT), using a commercial panel of allergenic extracts (Laboratorios LETI, Madrid, Spain). Allergic sensitisation was classified as seasonal when the subject was sensitised only to pollens or perennial when the participant had positive SPTs to dust mites, moulds and/or animals. Presence of positive SPTs to three or more aeroallergens was defined as multiple sensitisation. The absence of current asthma at the age of six years was ascertained by a specialist in paediatric pulmonology (SF), based on the following criteria: (a) no history of parent- or doctor-reported symptoms suggestive of asthma (wheezing, dyspnoea, relapsing cough) within the last two years, (b) no prescription of asthma-related medication within the last two years and, (c) normal spirometry at enrolment. A third specialist in paediatric allergy and pulmonology (KD) independently reviewed the data and confirmed that all participants fulfilled the eligibility criteria.
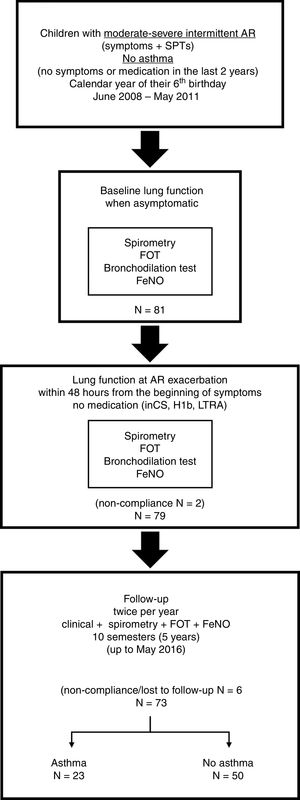
The study was approved by the ethics committee of the University Hospital of Patras, and written informed consent was obtained from the parents of the children prior to enrolment. The study flow is presented in Fig. 1.
Study protocolVisit 1: baseline lung function when asymptomaticEligible children were asked to visit our unit when they were completely free of AR and other respiratory symptoms for at least four weeks, and not receiving any relevant medication (i.e., intranasal corticosteroids, antihistamines, leukotriene receptor antagonists). After a thorough clinical examination, all children underwent a set of lung function measurements, performed in the following order: (a) measurement of exhaled nitric oxide fraction (FeNO) (NObreath, Bedfont Scientific, UK); (b) Measurement of respiratory impedance using a multifrequency (4–48Hz) pseudorandom noise FOT (i2m device, Chess Medical, Ghent, Belgium) according to standard recommendations.18 For each subject, the mean Rrs and Xrs at 6 and 8Hz of five technically acceptable measurements were recorded; (c) spirometry, performed using a MicroLab™ spirometer (CareFusion, San Diego, CA, USA). The best forced expiratory volume at 1s (FEV1), forced vital capacity (FVC) and forced expiratory flow between 25 and 75% of FVC (FEF25–75) of three technically acceptable measurements were recorded; and (d) Bronchial reversibility test, after administration of 300mcg of inhaled salbutamol (Aerolin™ 100mcg/dose, GlaxoSmithKline, Greece) via a holding chamber (Aerochamber™, Trudell Medical International, Canada). FOT followed by spirometric measurements were repeated 15min after bronchodilation (BD) and post-BD FEV1, FVC, FEF25–75, Rrs at 6 and 8Hz, and Xrs at 6 and 8Hz, were recorded.
Visit 2: lung function at AR exacerbationThe parents of the participants were instructed to bring their child to our unit at the first exacerbation of AR, within 48h from the beginning of the symptoms and prior to the administration of any relevant medication. Children who failed to present within 48h or received medication were excluded from the study. After the clinical confirmation of their symptoms, children underwent the same set of lung function measurements (and in the same order) as in visit 1. For each participant pre- and post-BD FEV1, FVC, FEF25–75, Rrs at 6 and 8Hz, and Xrs at 6 and 8Hz, were recorded.
Follow-up visitsAll children were asked to visit our unit twice per year, when completely asymptomatic for at least four weeks and not receiving any medication. At each visit, participants were carefully interviewed for the presence of symptoms suggestive of asthma or administration of asthma-related medication in the last semester, and underwent FeNO measurement, followed by measurement of respiratory impedance, and spirometry. Overall, 10 similar follow-up visits were planned for each participant, up to the age of 11 years. The development of asthma, as ascertained independently by two paediatric pulmonology specialists (KD and KB), was considered as the end-point of the study. Children who missed two consecutive visits (i.e., no follow-up during a year) and those who failed to attend our unit three or more times during the follow-up period, were excluded from the analysis.
Data collection and analysisAll data were stored in a database designated for the study. Raw FEV1, FVC, and FEF25–75 values were transformed in % predicted using the GLI-2012 Desktop Software for Large Data Sets, version 1.3.4.19 Bronchial reversibility was assessed by the relative % change of FEV1, FEF25–75, Rrs at 6 and 8 HZ, and Xrs at 6 and 8Hz after BD; for each parameter, the relative % change (Δ) from the baseline value was calculated as: (value post-BD−value pre-BD)×100/value pre-BD.
Differences in lung function between children who developed and those who did not develop asthma, were assessed by Student's t test. The ability of these parameters to predict the development of asthma was further explored by receiver operating characteristics (ROC) curve analysis, including assessment of the area under the ROC curve (AUC) and determination of the best discriminating value according to the highest Youden's J index (sensitivity+specificity−1).20
Based on the best discriminating cut-offs of Rrs and Xrs (see Results below), small-airway dysfunction (SAD) was defined as ΔRrs at 6 or 8Hz ≥ −30% and/or ΔXrs at 6 or 8Hz ≥ 50% after BD, irrespective of concurrent spirometric changes. The relationship between SAD at AR exacerbation and development of asthma was assessed by multivariable logistic regression analysis, after adjustment for the effect of other known risk factors, such as eczema, parental asthma and type of allergic sensitisation. Statistical analyses were carried out with the IBM SPSS software version 21.0 (IBM Corp., Armonk, NY, USA).
ResultsStudy populationEighty-one 6-year-old children with moderate-severe intermittent AR were initially recruited and performed baseline lung function when asymptomatic (visit 1). Two children were excluded from the study because they failed to comply to the requirements for measuring lung function during the exacerbation of AR (visit 2); both received medication (intranasal corticosteroids and antihistamines) prior to attending our unit. From the remaining 79 children, five failed to comply to the follow-up protocol (one child missed two consecutive follow-up visits; four children missed three or more follow-up visits) and were excluded from the analysis. None of these children developed asthma by the age of 11 years. One child was also excluded from the study due to relocation of his family to another city. Thus, the population included in the analysis consisted of 73 children with moderate-severe intermittent AR who were followed-up twice per year for five years up to May 2016 (Fig. 1).
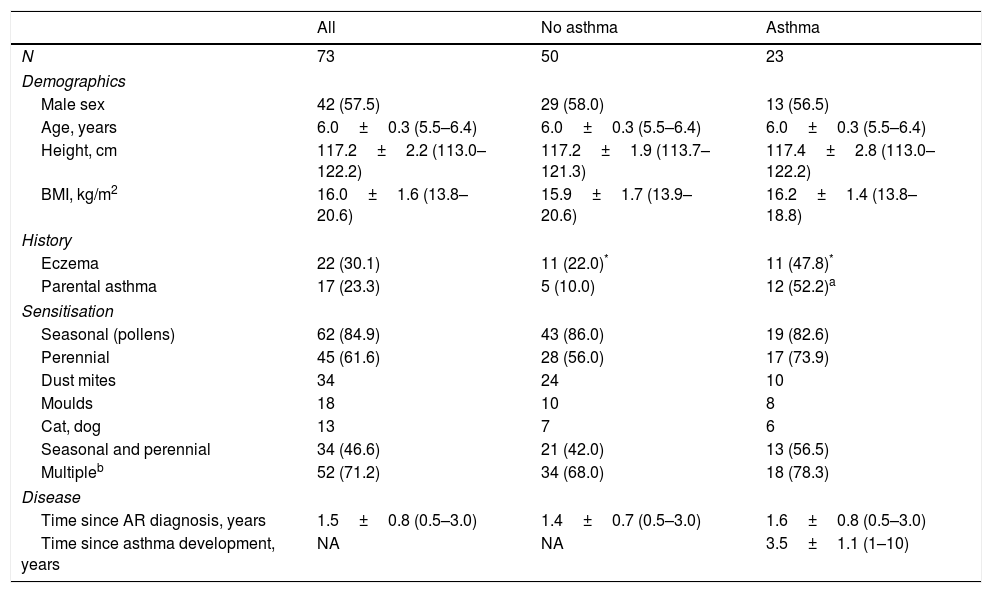
The characteristics of the study population are presented in Table 1. Twenty-three children (31.5%) developed asthma by the end of the follow-up period. There were no differences in demographics, type of sensitisation, and time elapsed since the diagnosis of AR between children who developed and those who did not develop asthma (Table 1). Participants who developed asthma were more likely to have eczema or at least one parent with diagnosed asthma (Table 1).
Population characteristics.
| All | No asthma | Asthma | |
|---|---|---|---|
| N | 73 | 50 | 23 |
| Demographics | |||
| Male sex | 42 (57.5) | 29 (58.0) | 13 (56.5) |
| Age, years | 6.0±0.3 (5.5–6.4) | 6.0±0.3 (5.5–6.4) | 6.0±0.3 (5.5–6.4) |
| Height, cm | 117.2±2.2 (113.0–122.2) | 117.2±1.9 (113.7–121.3) | 117.4±2.8 (113.0–122.2) |
| BMI, kg/m2 | 16.0±1.6 (13.8–20.6) | 15.9±1.7 (13.9–20.6) | 16.2±1.4 (13.8–18.8) |
| History | |||
| Eczema | 22 (30.1) | 11 (22.0)* | 11 (47.8)* |
| Parental asthma | 17 (23.3) | 5 (10.0) | 12 (52.2)a |
| Sensitisation | |||
| Seasonal (pollens) | 62 (84.9) | 43 (86.0) | 19 (82.6) |
| Perennial | 45 (61.6) | 28 (56.0) | 17 (73.9) |
| Dust mites | 34 | 24 | 10 |
| Moulds | 18 | 10 | 8 |
| Cat, dog | 13 | 7 | 6 |
| Seasonal and perennial | 34 (46.6) | 21 (42.0) | 13 (56.5) |
| Multipleb | 52 (71.2) | 34 (68.0) | 18 (78.3) |
| Disease | |||
| Time since AR diagnosis, years | 1.5±0.8 (0.5–3.0) | 1.4±0.7 (0.5–3.0) | 1.6±0.8 (0.5–3.0) |
| Time since asthma development, years | NA | NA | 3.5±1.1 (1–10) |
Data represent mean±SD (range) or number of cases (%).
Statistical significance calculated by Student's t or chi-square test, as appropriate.
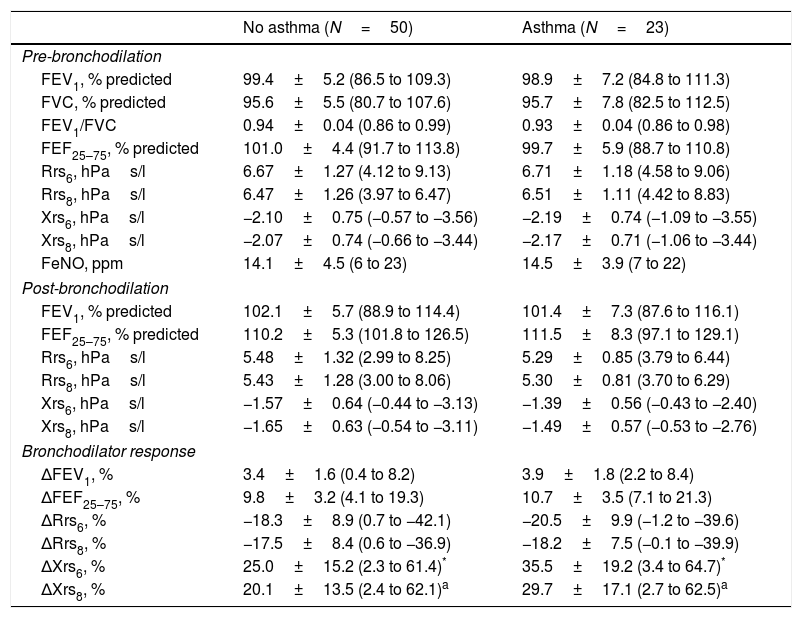
There were no differences in baseline lung function (pre- and post-BD) between children who developed and those who did not develop asthma. Baseline FeNO values were also similar (Table 2). The bronchodilator response, as assessed by ΔFEV1, ΔFEF25–75 or ΔRrs, did not differ between the two groups. Conversely, children who developed asthma had larger ΔXrs after bronchodilator administration (Table 2).
Baseline lung function.
| No asthma (N=50) | Asthma (N=23) | |
|---|---|---|
| Pre-bronchodilation | ||
| FEV1, % predicted | 99.4±5.2 (86.5 to 109.3) | 98.9±7.2 (84.8 to 111.3) |
| FVC, % predicted | 95.6±5.5 (80.7 to 107.6) | 95.7±7.8 (82.5 to 112.5) |
| FEV1/FVC | 0.94±0.04 (0.86 to 0.99) | 0.93±0.04 (0.86 to 0.98) |
| FEF25–75, % predicted | 101.0±4.4 (91.7 to 113.8) | 99.7±5.9 (88.7 to 110.8) |
| Rrs6, hPas/l | 6.67±1.27 (4.12 to 9.13) | 6.71±1.18 (4.58 to 9.06) |
| Rrs8, hPas/l | 6.47±1.26 (3.97 to 6.47) | 6.51±1.11 (4.42 to 8.83) |
| Xrs6, hPas/l | −2.10±0.75 (−0.57 to −3.56) | −2.19±0.74 (−1.09 to −3.55) |
| Xrs8, hPas/l | −2.07±0.74 (−0.66 to −3.44) | −2.17±0.71 (−1.06 to −3.44) |
| FeNO, ppm | 14.1±4.5 (6 to 23) | 14.5±3.9 (7 to 22) |
| Post-bronchodilation | ||
| FEV1, % predicted | 102.1±5.7 (88.9 to 114.4) | 101.4±7.3 (87.6 to 116.1) |
| FEF25–75, % predicted | 110.2±5.3 (101.8 to 126.5) | 111.5±8.3 (97.1 to 129.1) |
| Rrs6, hPas/l | 5.48±1.32 (2.99 to 8.25) | 5.29±0.85 (3.79 to 6.44) |
| Rrs8, hPas/l | 5.43±1.28 (3.00 to 8.06) | 5.30±0.81 (3.70 to 6.29) |
| Xrs6, hPas/l | −1.57±0.64 (−0.44 to −3.13) | −1.39±0.56 (−0.43 to −2.40) |
| Xrs8, hPas/l | −1.65±0.63 (−0.54 to −3.11) | −1.49±0.57 (−0.53 to −2.76) |
| Bronchodilator response | ||
| ΔFEV1, % | 3.4±1.6 (0.4 to 8.2) | 3.9±1.8 (2.2 to 8.4) |
| ΔFEF25–75, % | 9.8±3.2 (4.1 to 19.3) | 10.7±3.5 (7.1 to 21.3) |
| ΔRrs6, % | −18.3±8.9 (0.7 to −42.1) | −20.5±9.9 (−1.2 to −39.6) |
| ΔRrs8, % | −17.5±8.4 (0.6 to −36.9) | −18.2±7.5 (−0.1 to −39.9) |
| ΔXrs6, % | 25.0±15.2 (2.3 to 61.4)* | 35.5±19.2 (3.4 to 64.7)* |
| ΔXrs8, % | 20.1±13.5 (2.4 to 62.1)a | 29.7±17.1 (2.7 to 62.5)a |
Data are means±SD with ranges in parentheses.
Statistical significance calculated by Student's t test.
P=0.019.
FEV1, forced expiratory volume at 1s; FVC, forced vital capacity; FEF25–75, forced expiratory flow between 25 and 75% of FVC; Rrs6, respiratory resistance at 6Hz; Rrs8, respiratory resistance at 8Hz; Xrs6, respiratory reactance at 6Hz; Xrs8, respiratory reactance at 8Hz; Δ, % change after administration of 400mcg of salbutamol inhaler.
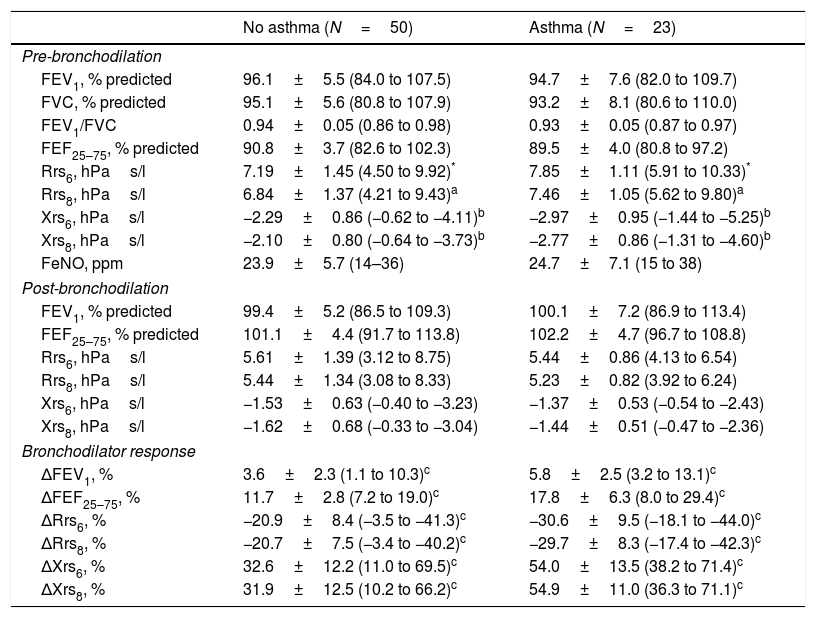
At AR exacerbation, there were no differences in spirometric parameters or FeNO levels prior to BD between children who developed asthma and those who did not (Table 3). Conversely, children who developed asthma had higher Rrs and lower Xrs values prior to BD (Table 3). Post-BD spirometric and FOT parameters were similar between the two groups (Table 3). At AR exacerbation, children who developed asthma had significantly larger ΔFEV1, ΔFEF25–75, ΔRrs and ΔXrs after bronchodilator administration compared to controls (Table 3).
Lung function at AR exacerbation.
| No asthma (N=50) | Asthma (N=23) | |
|---|---|---|
| Pre-bronchodilation | ||
| FEV1, % predicted | 96.1±5.5 (84.0 to 107.5) | 94.7±7.6 (82.0 to 109.7) |
| FVC, % predicted | 95.1±5.6 (80.8 to 107.9) | 93.2±8.1 (80.6 to 110.0) |
| FEV1/FVC | 0.94±0.05 (0.86 to 0.98) | 0.93±0.05 (0.87 to 0.97) |
| FEF25–75, % predicted | 90.8±3.7 (82.6 to 102.3) | 89.5±4.0 (80.8 to 97.2) |
| Rrs6, hPas/l | 7.19±1.45 (4.50 to 9.92)* | 7.85±1.11 (5.91 to 10.33)* |
| Rrs8, hPas/l | 6.84±1.37 (4.21 to 9.43)a | 7.46±1.05 (5.62 to 9.80)a |
| Xrs6, hPas/l | −2.29±0.86 (−0.62 to −4.11)b | −2.97±0.95 (−1.44 to −5.25)b |
| Xrs8, hPas/l | −2.10±0.80 (−0.64 to −3.73)b | −2.77±0.86 (−1.31 to −4.60)b |
| FeNO, ppm | 23.9±5.7 (14–36) | 24.7±7.1 (15 to 38) |
| Post-bronchodilation | ||
| FEV1, % predicted | 99.4±5.2 (86.5 to 109.3) | 100.1±7.2 (86.9 to 113.4) |
| FEF25–75, % predicted | 101.1±4.4 (91.7 to 113.8) | 102.2±4.7 (96.7 to 108.8) |
| Rrs6, hPas/l | 5.61±1.39 (3.12 to 8.75) | 5.44±0.86 (4.13 to 6.54) |
| Rrs8, hPas/l | 5.44±1.34 (3.08 to 8.33) | 5.23±0.82 (3.92 to 6.24) |
| Xrs6, hPas/l | −1.53±0.63 (−0.40 to −3.23) | −1.37±0.53 (−0.54 to −2.43) |
| Xrs8, hPas/l | −1.62±0.68 (−0.33 to −3.04) | −1.44±0.51 (−0.47 to −2.36) |
| Bronchodilator response | ||
| ΔFEV1, % | 3.6±2.3 (1.1 to 10.3)c | 5.8±2.5 (3.2 to 13.1)c |
| ΔFEF25–75, % | 11.7±2.8 (7.2 to 19.0)c | 17.8±6.3 (8.0 to 29.4)c |
| ΔRrs6, % | −20.9±8.4 (−3.5 to −41.3)c | −30.6±9.5 (−18.1 to −44.0)c |
| ΔRrs8, % | −20.7±7.5 (−3.4 to −40.2)c | −29.7±8.3 (−17.4 to −42.3)c |
| ΔXrs6, % | 32.6±12.2 (11.0 to 69.5)c | 54.0±13.5 (38.2 to 71.4)c |
| ΔXrs8, % | 31.9±12.5 (10.2 to 66.2)c | 54.9±11.0 (36.3 to 71.1)c |
Data are means±SD with ranges in parentheses.
Statistical significance calculated by Student's t test.
P<0.001.
FEV1, forced expiratory volume at 1s; FVC, forced vital capacity; FEF25–75, forced expiratory flow between 25 and 75% of FVC; Rrs6, respiratory resistance at 6Hz; Rrs8, respiratory resistance at 8Hz; Xrs6, respiratory reactance at 6Hz; Xrs8, respiratory reactance at 8Hz.
Δ, % change after administration of 400mcg of salbutamol inhaler.
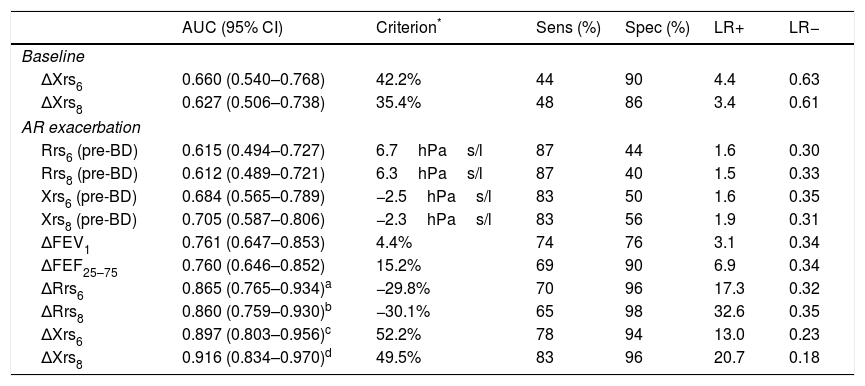
The predictive ability of baseline ΔXrs and of pre-BD Rrs and Xrs at AR exacerbation was low, while that of ΔFEV1 and ΔFEF25–75 at AR exacerbation was intermediate (Table 4). The predictive characteristics of bronchodilator response, as assessed by FOT parameters at AR exacerbation, were high; ΔRrs and ΔXrs at 6 and 8Hz performed better than ΔFEV1 in predicting the development of asthma (Table 4). The predictive ability of ΔXrs at 6 and 8Hz was also better than that of ΔFEF25–75 at AR exacerbation (Table 4).
Predictive ability of lung function parameters for development of asthma.
| AUC (95% CI) | Criterion* | Sens (%) | Spec (%) | LR+ | LR− | |
|---|---|---|---|---|---|---|
| Baseline | ||||||
| ΔXrs6 | 0.660 (0.540–0.768) | 42.2% | 44 | 90 | 4.4 | 0.63 |
| ΔXrs8 | 0.627 (0.506–0.738) | 35.4% | 48 | 86 | 3.4 | 0.61 |
| AR exacerbation | ||||||
| Rrs6 (pre-BD) | 0.615 (0.494–0.727) | 6.7hPas/l | 87 | 44 | 1.6 | 0.30 |
| Rrs8 (pre-BD) | 0.612 (0.489–0.721) | 6.3hPas/l | 87 | 40 | 1.5 | 0.33 |
| Xrs6 (pre-BD) | 0.684 (0.565–0.789) | −2.5hPas/l | 83 | 50 | 1.6 | 0.35 |
| Xrs8 (pre-BD) | 0.705 (0.587–0.806) | −2.3hPas/l | 83 | 56 | 1.9 | 0.31 |
| ΔFEV1 | 0.761 (0.647–0.853) | 4.4% | 74 | 76 | 3.1 | 0.34 |
| ΔFEF25–75 | 0.760 (0.646–0.852) | 15.2% | 69 | 90 | 6.9 | 0.34 |
| ΔRrs6 | 0.865 (0.765–0.934)a | −29.8% | 70 | 96 | 17.3 | 0.32 |
| ΔRrs8 | 0.860 (0.759–0.930)b | −30.1% | 65 | 98 | 32.6 | 0.35 |
| ΔXrs6 | 0.897 (0.803–0.956)c | 52.2% | 78 | 94 | 13.0 | 0.23 |
| ΔXrs8 | 0.916 (0.834–0.970)d | 49.5% | 83 | 96 | 20.7 | 0.18 |
Significantly better than ΔFEV1 (P=0.003) and ΔFEF25–75 (P=0.016).
AUC, area under the receiver operating characteristics (ROC) curve; Sens, sensitivity; Spec, specificity; LR+, positive likelihood ratio; LR−, negative likelihood ratio; AR, allergic rhinitis; BD, bronchodilation (400mcg salbutamol inhaler).
FEV1, forced expiratory volume at 1s; FEF25–75, forced expiratory flow between 25 and 75% of FVC; Rrs6, respiratory resistance at 6Hz; Rrs8, respiratory resistance at 8Hz; Xrs6, respiratory reactance at 6Hz; Xrs8, respiratory reactance at 8Hz; Δ, % parameter change after BD.
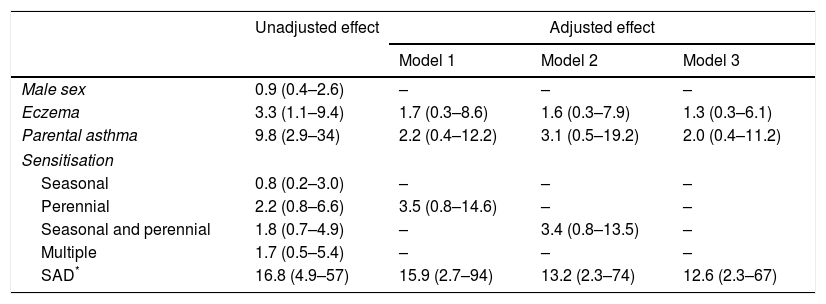
SAD at AR exacerbation, personal history of eczema, and parental asthma were all significant predictors of subsequent asthma when their effect was assessed separately (univariable logistic regression analysis) (Table 5). Conversely, the effect of the type of sensitisation (seasonal, perennial, seasonal and perennial, multiple) was not significant (Table 5). When the effects of SAD, eczema, and parental asthma were adjusted for each other (various multivariable logistic regression models) SAD at AR exacerbation emerged as the only significant and independent predictor of the development of asthma. Notably, the effect of both parental asthma and eczema was masked by the effect of SAD (Table 5).
Predictors of asthma development in children with AR.
| Unadjusted effect | Adjusted effect | |||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | ||
| Male sex | 0.9 (0.4–2.6) | – | – | – |
| Eczema | 3.3 (1.1–9.4) | 1.7 (0.3–8.6) | 1.6 (0.3–7.9) | 1.3 (0.3–6.1) |
| Parental asthma | 9.8 (2.9–34) | 2.2 (0.4–12.2) | 3.1 (0.5–19.2) | 2.0 (0.4–11.2) |
| Sensitisation | ||||
| Seasonal | 0.8 (0.2–3.0) | – | – | – |
| Perennial | 2.2 (0.8–6.6) | 3.5 (0.8–14.6) | – | – |
| Seasonal and perennial | 1.8 (0.7–4.9) | – | 3.4 (0.8–13.5) | – |
| Multiple | 1.7 (0.5–5.4) | – | – | – |
| SAD* | 16.8 (4.9–57) | 15.9 (2.7–94) | 13.2 (2.3–74) | 12.6 (2.3–67) |
Regression coefficients beta calculated by univariable (unadjusted effect) and multivariable (adjusted effect) logistic regression analysis.
In this study, we explored the existence of subclinical lung function changes in six-year-old children perceiving only intermittent symptoms of moderate-severe AR, and we assessed the ability of these functional abnormalities to predict the development of asthma within the next five years. We showed that, in the absence of nasal symptoms, there were no differences in lung function between children who developed and those who did not develop asthma. However, an exaggerated bronchodilator response at AR exacerbation, as expressed primarily by means of respiratory impedance (ΔRrs and ΔXrs at 6 and 8Hz), was noted in the group of children who eventually developed asthma. The predictive characteristics of ΔRrs and ΔXrs were exceptional and superior to those of concomitant spirometric changes. The presence of subclinical SAD, as expressed by the above abnormalities in respiratory impedance, emerged as the single most efficient predictor of subsequent asthma, independently of other risk factors, such as parental asthma, personal history of eczema and type of allergic sensitisation.
Small-airway dysfunction in the absence of asthmatic symptoms is a known feature of AR.11,15 For example, Capasso et al.11 showed that non-asthmatic children with AR (age range 8–16 years) were more likely to present bronchial reversibility to BD (ΔFEV1 >12%) compared to healthy controls, and that the magnitude of bronchodilator response was related to the duration of AR and the sensitisation to perennial allergens. Saranz et al.15 confirmed the existence of subclinical spirometric changes in non-asthmatic children with AR (age range 5–18 years), and showed that the impairment of lung function was more common in the persistent and more severe forms of the disease.
Despite these cross-sectional observations, existing evidence on whether subclinical lung function abnormalities can predict the progression from AR to asthma remains sparse. An early small study concluded that non-asthmatic children with AR (age range 6–14 years) who developed asthma within four years from the enrolment did not show significant lung function differences as compared to controls.16 Conversely, a large longitudinal study using cluster analysis,17 demonstrated that non-asthmatic children (age range 6–8 years) with AR and reduced FEV1 and/or FEF25–75 were more likely to develop bronchial hyper-reactivity and asthma within a follow-up period of four years.
To the best of our knowledge, the present study is the first longitudinal survey to explore the implications of SAD in non-asthmatic children with AR by means of respiratory impedance. Kim et al.,14 compared the magnitude of bronchial hyper-responsiveness (methacholine challenge test) and the bronchodilator response (determined by both spirometry and FOT) between asthmatic children with AR, non-asthmatic children with AR (interquartile range of age 7.9–11.1 years) and healthy controls. They found that ΔXrs at 5Hz, as well as post-BD changes in the reactance area, performed significantly better than the measurement of bronchial hyper-reactivity, ΔFEV1, and ΔFEF25–75 in detecting small-airway involvement. Despite the cross-sectional nature of their data, the authors suggest that bronchial reversibility determined by means of respiratory impedance may be a more useful tool in predicting the progression from AR to asthma compared to spirometry.14 The findings of our study provide sound basis to the above concept.
The FOT has emerged as a valuable tool for evaluating lung function in younger or less cooperative children.18 More importantly, it has been shown that specific indices of respiratory impedance, such as Rrs and Xrs at low frequencies (i.e., 4–8Hz), are particularly sensitive in identifying subtle lung function changes in various pulmonary disorders.21,22 The method has been also proven superior to spirometry in detecting small-airway involvement in children at risk for persistent asthma23–25 and in those with exercise-induced dyspnoea but a negative or borderline exercise challenge test.26 In this context, it is not surprising that post-BD changes in respiratory impedance performed better than the spirometric parameters in predicting the development of asthma in our cohort.
Small-airway dysfunction (traditionally defined as decreased FEF25–75 values with an otherwise normal spirometry)27 has been recognised as an early marker of bronchial involvement in non-asthmatic individuals with AR.10 However, it has been also shown that the extent of spirometric impairment in these patients is related to the duration of allergic inflammation and the frequency of exposure to causal allergens.28–30 In contrast to previous studies, our survey included solely children with intermittent nasal symptoms who at the time of enrolment were younger and, therefore, less exposed to allergic inflammation over time; this may explain why significant bronchial reversibility was detected only by means of respiratory impedance and only at AR exacerbation in our cohort. Notably, natural exposure to inhalant allergens has been shown to increase bronchial reactivity in individuals with seasonal rhinitis.31 In the present study, therefore, the exacerbation of AR served as a natural “challenge” which was anticipated to enhance airway reactivity and facilitate the detection of SAD.
We studied solely children with moderate-severe intermittent AR according to the ARIA classification.8,9 However, emerging evidence suggests that both persistence and severity of AR are closely related to the development of asthma.2,7 In a recent study by Di Cara et al.,32 all non-asthmatic children with moderate-severe persistent AR progressed to asthma within a five-year period, while only 33% of their counterparts with mild persistent AR and 6.8% of those with intermittent symptoms had a similar outcome. Of note, in the 61.5% of those who developed asthma in the above survey, the clinical characteristics of the disease (i.e., persistence and/or severity) worsened during the follow-up period.32 The design of our study (i.e., only inclusion of children with moderate-severe intermittent symptoms) does not permit speculation on the effect of less severe or more persistent forms of AR. In a subgroup of our cohort, the clinical characteristics of AR worsened during the five-year follow-up period (data not shown); however, the assessment of the contribution of this phenomenon to the development of asthma, especially in relation to parallel changes in lung function,15 was not within the objectives of the present report.
It is also true that the reported data derive from a single population of Greek children and from a limited geographical area. However, the type of allergic sensitisation and the clinical characteristics of AR may present significant variations due to geographical and climatic parameters, even within the same ethnic group and within the same country.33,34 Therefore, although the allergic and clinical profiles of our participants were comparable to those reported from other areas of Greece and more or less-representative for the Mediterranean region,35 our results may not be generalisable to other populations or different settings.
Measurement of FeNO levels has emerged as a standard method for the assessment of bronchial inflammation in AR.36 It has been shown that allergic children with nasal symptoms have higher FeNO values than those with non-allergic rhinitis.37 More importantly, among non-asthmatic children with AR, those who present bronchial hyper-reactivity also express higher FeNO level,14 suggesting that FeNO may be an indirect marker of early bronchial involvement in these patients.10,14 We did not observe significant FeNO differences between children who progressed and of those who did not progress to asthma, which may be attributed to the aforementioned peculiarities of our cohort (i.e., young age, intermittent symptoms). However, given the concomitant marked differences in the FOT measurements, it is tempting to speculate that SAD precedes the establishment of airway inflammation and/or hyper-reactivity and, therefore, reflects an early stage of progression from AR to asthma10; since we lack direct measurements of airway inflammation and bronchial hyper-reactivity, this hypothesis requires confirmation in future studies.
In conclusion, this prospective longitudinal study provides evidence that school-aged children with intermittent moderate-severe AR, may present subclinical lung function abnormalities which are primarily expressed by significant post-BD changes in respiratory impedance. These subtle abnormalities, which are indicative of SAD, become particularly evident during the exacerbation of AR and are predictive of the subsequent development of asthma. We suggest that the detection of SAD by means of respiratory impedance in allergic school-aged children who perceive only nasal symptoms, may assist in identifying those who are at risk of developing asthma and, thus, in planning targeted follow-up strategies for these patients.
Funding sourceNone.
Conflict of interestThe authors have no conflict of interest to declare.