The sensitisation profile at molecular level in plant-food allergy is complex. Several allergens may be involved, with different potential for severe reactions. lipid transfer proteins (LTP) are considered the most relevant plant-food allergens in adults in Mediterranean countries, but less is known in children.
AimTo describe the clinical pattern and sensitisation profile of children with plant-food allergy and LTP sensitisation from Northeast Spain.
MethodsChildren with history of immediate reaction to plant-food(s), positive skin-prick-test to the culprit plant-food(s) and specific-IgE to plant-food LTPs were analysed.
Results130 children were included. 69.2% (90/130) had reacted to ≥2 taxonomically unrelated plant-foods. Peach, walnut, hazelnut and peanut were most frequently involved. Reactions severity ranged from anaphylaxis (45.4%, 59/130) to oral symptoms only. Sensitisation to a particular plant-food LTP not always caused clinical symptoms with that plant-food; 69% (40/58) and 63% (17/27) of peach- and walnut-tolerant subjects had positive rPru p 3 and nJug r 3 specific IgE, respectively. 65.4% (85/130) of children were also sensitised to storage proteins, which was associated to anaphylaxis and nut allergy. However, 60% of patients without nuts/seeds allergy were sensitised to storage proteins. Specific-IgE levels to LTPs and/or storage proteins were not useful to predict allergy (vs. tolerance) to peach, walnut, peanut or hazelnut.
ConclusionsSensitisation to LTP and/or storage proteins without clear clinical significance is relatively common. Prospective longitudinal studies are required to evaluate the relevance of these silent sensitisations over time. Caution is required when interpreting the results of molecular-based diagnostic tools in clinical practice.
The sensitisation profile at a molecular level in plant food allergy is complex. Several allergens may be involved, which imply different potential for reaction severity.1–4 Bet v 1 homologues and profilins are mainly involved in pollen-food allergy syndrome, which in most cases consists of exclusively oral symptoms due to a cross-reactivity phenomenon with pollen proteins.5–7 In contrast, storage proteins and lipid transfer proteins (LTP) have been traditionally associated with the potential to trigger systemic more severe reactions.8–10 LTPs are considered the most relevant family of plant food allergens in Mediterranean countries.11
The potential of multiplexed molecular-based diagnostic tools, such as the ImmunoCAP ISAC microarray, to accurately characterise plant-food allergic patients and help clinicians in individual risk assessment has led to an increase in its use in clinical practice.12,13 However, certain observations make results interpretation complex and challenging for the clinician.14,15 For instance, in LTP-sensitised patients allergic manifestations can range from mild symptoms to severe anaphylaxis.11 The reason for such variability is unclear and only partially explained by LTP-specific immunoglobulin E (sIgE) levels.16–18 In addition, the range of plant-foods involved can be extremely wide, including clinical reactivity to several taxonomically unrelated plant-foods in an individual, whereas others closely related are tolerated. Likewise, sensitisation to a wide panel of plant-foods due to cross-reactivity (either by skin prick test or specific IgE) is common in LTP-sensitised patients, although its clinical significance is not always clear.18–20 Finally, most evidence comes from studies in adult populations, whereas less is known about LTP sensitisation/allergy in children.
In this context, we sought to describe the clinical pattern and sensitisation profile of children with plant-food allergy and LTP sensitisation from our Mediterranean area in Spain. In a previous study21 we described a cohort of adults from the same geographical area with LTP syndrome, i.e. clinical reactivity to taxonomically unrelated plant-foods which was exclusively explained by LTP sensitisation, in the absence of sensitisation to other plant-food allergens. Anaphylaxis and symptoms exacerbation by cofactors such as exercise or non-steroidal anti-inflammatory drugs were common. We hypothesised that a similar clinical and sensitisation profile might be found in children.
MethodsStudy profile/designPatients aged 0–18 years from the Barcelona area, Spain, diagnosed of plant-food allergy by (a) a convincing history of immediate allergic reactions to plant-foods (i.e., objective signs or repeated subjective symptoms to a particular food on several occasions), plus (b) a positive skin-prick-test (SPT, defined as the presence of a wheal with a mean diameter ≥3mm as per European Standards22) to the culprit plant-food/s extract/s (ALK-Abelló, Spain), and (c) plant-food LTP sensitisation detected by microarray (ImmunoCAPISAC™ 112, ThermoFisher Scientific, USA) were recruited (cut-off: ≥0.3 ISAC Standardised Unit, ISU, as per manufacturer's recommendation). rPru p 3 sIgE was also determined by ImmunoCAP (ThermoFisher Scientific; cut-off: ≥0.35kUA/L). Medical records were retrospectively reviewed and data on plant-foods involved in the reaction and its clinical presentation (urticaria/angioedema (U/AE), oropharyngeal symptoms (OS), gastrointestinal symptoms (i.e. nausea, abdominal pain or vomiting), respiratory symptoms and/or anaphylaxis) collected. Anaphylaxis was defined as per the relevant EAACI position paper, i.e., respiratory or cardiovascular compromise or persistent gastrointestinal symptoms in association with skin symptoms.23 Data on tolerance to peach, walnut, hazelnut, peanut and wheat were collected from medical records (since their LTPs are included in the microarray panel of allergens). Tolerance was defined as no reactivity to age-appropriate portions of the food in patients’ usual diet. Clinical data were re-checked and completed prospectively in a subsequent appointment in all cases. Sensitisation to other plant-food panallergens included in the microarray (storage proteins, PR-10, proteins and profilins) was also evaluated. Written informed consent was obtained. The Local Ethics Committee approved the study.
StatisticsQualitative variables were described in absolute frequencies and percentages. Quantitative variables were described by mean and standard deviation (SD) for parametric data and median and range for non-parametric data. Sensitisation and sIgE levels to LTPs and storage-proteins were compared between patients with and without allergy to peach, walnut, hazelnut and peanut, as well as between patients with and without anaphylaxis to each plant-food. Reaction severity (i.e. anaphylaxis rate) was compared in patients exclusively sensitised to LTP versus those also sensitised to storage proteins or to other plant-food panallergens. Parametrical and non-parametrical tests were used for comparative analysis as appropriate. The diagnostic performance of sIgE to LTPs and storage-proteins to discriminate tolerant versus allergic individuals was evaluated by receiver operating characteristic (ROC) curve analysis. PASW Statistics 18 (SPSS Inc., Chicago, IL, USA) was used. A two-tailed p value <0.05 was considered significant.
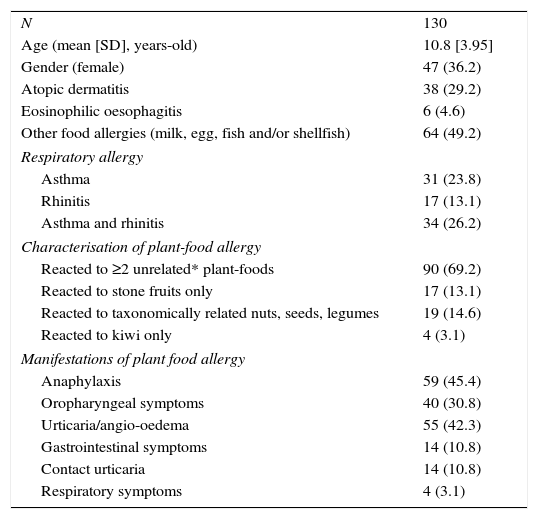
ResultsA total of 130 patients were recruited for the present study. The mean age was 10.8 years (SD; range: 3.95; 3–18). Demographics and other allergic co-morbidities of patients recruited are described in Table 1.
Characteristics subjects recruited.
| N | 130 |
| Age (mean [SD], years-old) | 10.8 [3.95] |
| Gender (female) | 47 (36.2) |
| Atopic dermatitis | 38 (29.2) |
| Eosinophilic oesophagitis | 6 (4.6) |
| Other food allergies (milk, egg, fish and/or shellfish) | 64 (49.2) |
| Respiratory allergy | |
| Asthma | 31 (23.8) |
| Rhinitis | 17 (13.1) |
| Asthma and rhinitis | 34 (26.2) |
| Characterisation of plant-food allergy | |
| Reacted to ≥2 unrelated* plant-foods | 90 (69.2) |
| Reacted to stone fruits only | 17 (13.1) |
| Reacted to taxonomically related nuts, seeds, legumes | 19 (14.6) |
| Reacted to kiwi only | 4 (3.1) |
| Manifestations of plant food allergy | |
| Anaphylaxis | 59 (45.4) |
| Oropharyngeal symptoms | 40 (30.8) |
| Urticaria/angio-oedema | 55 (42.3) |
| Gastrointestinal symptoms | 14 (10.8) |
| Contact urticaria | 14 (10.8) |
| Respiratory symptoms | 4 (3.1) |
Demographics and clinical characteristics of the patients’ studied, absolute numbers and percentages in brackets. (*) based on the Sociedad Española Inmunología Clínica, Alergología y Asma Pediátrica (SEICAP) food allergens classification. SD: standard deviation.
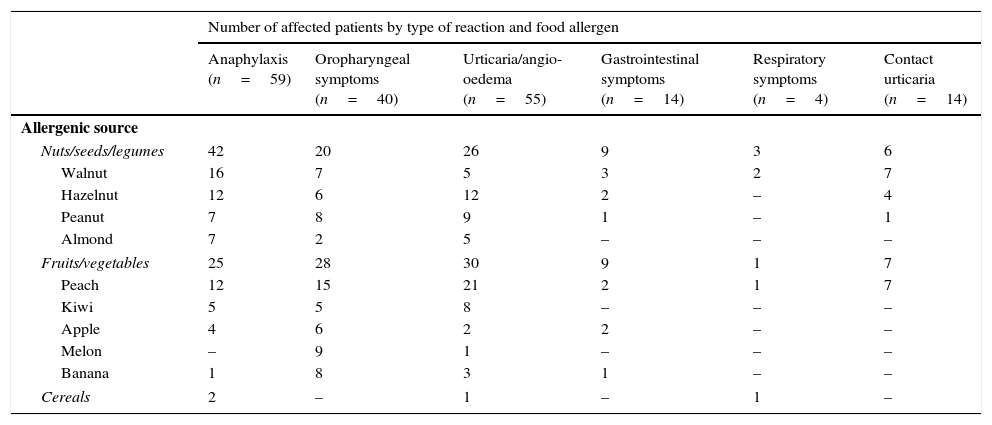
A broad diversity of culprit plant foods was observed; involving both taxonomically related and unrelated fruits, vegetables, nuts, seeds and legumes (Table 2). Most patients had suffered several allergic reactions of different type and the median number of culprit plant-foods involved per patient was two (range: 1–11). Briefly, 69.2% (90/130) of the patients had had an immediate allergic reaction to ≥2 unrelated plant-foods (according to the botanical taxonomic classification24), 13.1% (17/130) to stone-fruits only, 14.6% (19/130) to several taxonomically-related nuts, seeds or legumes and 3.1% (4/130) to kiwi. Globally, 73% of the patients (95/130) had a history of having reacted at least to one nut. The four most frequent plant-foods involved in reactions (considering any type of reaction) were: peach (N=58 patients), walnut (N=40), hazelnut (N=26) and peanut (N=26) (Table 2).
Main plant-foods involved and associated clinical symptoms.
| Number of affected patients by type of reaction and food allergen | ||||||
|---|---|---|---|---|---|---|
| Anaphylaxis (n=59) | Oropharyngeal symptoms (n=40) | Urticaria/angio-oedema (n=55) | Gastrointestinal symptoms (n=14) | Respiratory symptoms (n=4) | Contact urticaria (n=14) | |
| Allergenic source | ||||||
| Nuts/seeds/legumes | 42 | 20 | 26 | 9 | 3 | 6 |
| Walnut | 16 | 7 | 5 | 3 | 2 | 7 |
| Hazelnut | 12 | 6 | 12 | 2 | – | 4 |
| Peanut | 7 | 8 | 9 | 1 | – | 1 |
| Almond | 7 | 2 | 5 | – | – | – |
| Fruits/vegetables | 25 | 28 | 30 | 9 | 1 | 7 |
| Peach | 12 | 15 | 21 | 2 | 1 | 7 |
| Kiwi | 5 | 5 | 8 | – | – | – |
| Apple | 4 | 6 | 2 | 2 | – | – |
| Melon | – | 9 | 1 | – | – | – |
| Banana | 1 | 8 | 3 | 1 | – | – |
| Cereals | 2 | – | 1 | – | 1 | – |
The absolute number refers to the number of children affected by each type of allergic reaction (anaphylaxis, oropharyngeal symptoms, urticaria/angio-oedema, gastrointestinal symptoms, respiratory symptoms and contact urticaria) among analysed patients (n=130). Most patients had suffered from several allergic reactions to several foods and they had presented with different type of symptoms in different reactions. The main plant-foods involved in reactions are presented in the table, but other plant-foods causing reactions less commonly were (number of patients affected in brackets): lentil (10), chickpea (9), plum (8), lettuce (6), apricot/tomato/pear (5), green-bean/pineapple/soy/wheat/watermelon/soy/cherry (4), pistachio/chestnut/pea/white-bean/sunflower-seed/strawberry/cashew (3), sesame/Paraguayan/pinenut//nectarine/mustard (2), nutmeg/potato/rice/courgette/khaki/corn/grapes/lupin/pomegranate/butterbean/carrot (1).
A heterogeneous pattern of allergic manifestations was observed (Tables 1 and 2) from anaphylaxis in 45.4% (59/130) of the patients to skin symptoms exclusively (i.e. hives, angio-oedema, pruritus, rash or erythema) in 42.3% (55/130). Globally, fruits/vegetables elicited anaphylaxis less frequently than nuts/seeds/legumes (in 24 patients versus 41 patients, respectively; p<0.05). Anaphylaxis was mainly caused by walnut (16 patients), hazelnut (12), peach (12), peanut (7) and almond (7) (Table 2).
Intense physical exercise was reported to be associated with the reaction only in three patients (aged 8, 11 and 14, respectively) with a total of five episodes (two patients’ experienced two episodes each). Three of these episodes consisted of anaphylaxis and involved two patients. The third case experienced urticaria only. Plant-foods involved in these cases were apple, green bean, potato and lettuce and they had been tolerated so far in those patients in absence of exercise. No other cofactors (NSAID, menstruation) were reflected in medical records for the rest of the patients included in the study.
Sensitisation pattern to LTPsAll patients were sensitised to at least one plant-food LTP, as per inclusion criteria (Fig. E1 Online-Repository). Prevalence of LTP sensitisation detected by microarray was: 83.1% (108/130) peach-LTP (rPru p 3), 77.7% (101/130) walnut-LTP (nJug r 3), 56.2% (73/130) peanut-LTP (rAra h 9), 55.4% (72/130) hazelnut-LTP (rCor a 8) and 26.2% (34/130) wheat-LTP (rTri a 14). 96.9% (126/130) of patients were found to be sensitised to rPru p 3 by ImmunoCAP.
In 69.3% of the patients (90/130) pollen LTPs were positive (nArt v 3: 50.8% (66/130), rPla a 3: 60.8% (79/130), nOle e 7: 24.6% (32/130) and/or rPar j 2: 14.6% (19/130)).
Sensitisation to nJug r 3 showed strong association with sensitisation to rPru p 3 (i.e., 88% of nJug r 3-positive patients were also rPru p 3-positive). Sensitisation to rAra h 9 and rCor a 8 occurred exclusively or almost exclusively (in 93.1–100% of cases) in rPru p 3 and/or nJug r 3-positive individuals. A strong association was also observed between sensitisation to rCor a 8 and/or rAra h 9 and sensitisation to the pollen-LTPs rPla a 3 and nArt v 3 (77.8–90.4%) (Table E1-Online Repository).
In terms of diversity of LTPs sensitisation, subjects sensitised to pollen LTPs (n=90), compared to those who were not (n=40), had positive sIgE to a broader number of plant-food LTPs. Indeed, sensitisation to all the plant-food LTPs tested occurred in 70% (63/90) of the patients with pollen LTPs sensitisation vs. 2.5% (1/40) of the patients without pollen LTPs sensitisation. In contrast, sensitisation to only one plant-food LTP occurred in 6.7% (6/90) vs. 57.5% (23/40) of patients of each subgroup, respectively; p<0.001). However, no differences were found in the number of plant-foods involved in allergic reactions among those with pollen-LTP sensitisation and those without (mean: 3 vs. 2.4 culprit plant-foods, p=0.128). Equivalent findings were observed when each pollen LTP was analysed individually with regards to the number of plant-food LTP sensitisations and the number of plant-food allergies (data not shown).
Sensitisation to other plant-food panallergensAlong with LTP, 65.4% (85/130) of patients were co-sensitised to storage-proteins, whereas only 18.5% (24/130) to profilin and 12.3% (16/130) to PR-10 (Sensitisation rates to each specific panallergen tested are presented in Table E2-Online Repository). 5.38% (7/130) of patients were sensitised to rPru p 1, along with a sensitistion to rPru p 3.
Assessment of potential clinical relevance of plant-food sensitisations detected by ImmunoCAP ISACAnaphylaxisSensitistion to storage proteins was associated to experiencing anaphylaxis in the community (51.7% (46/89) of cases, whereas only 31.7% (13/41) of patients sensitised to LTP but not to storage proteins did so (p=0.042)). In contrast, the following co-sensitisations were not associated with anaphylaxis: co-sensitisation to pollen-LTPs (considering any pollen LTP, p=0.617; or considering the individual pollen LTPs separately: nArt v 3, p: 0.886; nOle e 7, p=0.831; rPla a 3: 0.911), profilins (p=0.563), PR-10 (p=0.953) or polcalcins (p=0.156). Co-sensitisation to rPrup 1 alongside with rPru p 3 was not associated with anaphylaxis (p=0.398).sIgE levels to plant-food panallergens were not different in allergic children with previous anaphylactic reactions versus those with non-anaphylactic reactions to peach, walnut or peanut. However, for hazelnut allergy sIgE levels to nCor a 9 were higher in children with hazelnut-induced anaphylaxis vs hazelnut-allergic non-anaphylactic children (median: 0.25 (IQR: 0.025–2.35) vs. 0 (IQR: 0–0.09), p=0.0004).
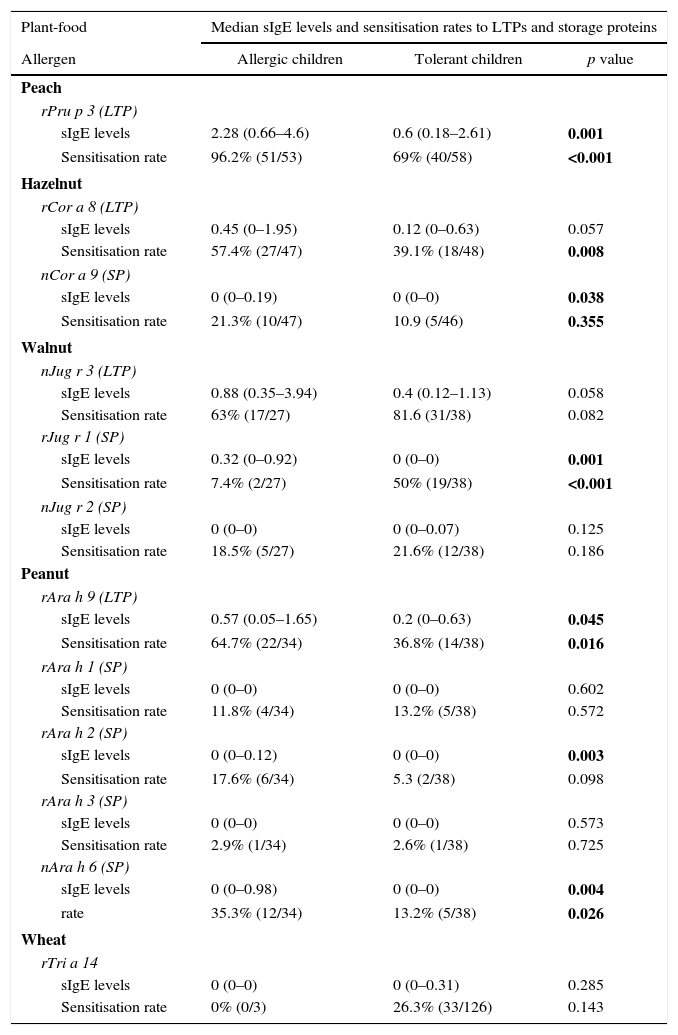
Allergy versus toleranceThe specific IgE levels and sensitisation rates to plant-food LTPs and storage proteins in children with allergy versus tolerance to peach, peanut, walnut and hazelnut are shown in Table 3.
Specific IgE levels and sensitisation rates to LTPs and storage proteins.
| Plant-food | Median sIgE levels and sensitisation rates to LTPs and storage proteins | ||
|---|---|---|---|
| Allergen | Allergic children | Tolerant children | p value |
| Peach | |||
| rPru p 3 (LTP) | |||
| sIgE levels | 2.28 (0.66–4.6) | 0.6 (0.18–2.61) | 0.001 |
| Sensitisation rate | 96.2% (51/53) | 69% (40/58) | <0.001 |
| Hazelnut | |||
| rCor a 8 (LTP) | |||
| sIgE levels | 0.45 (0–1.95) | 0.12 (0–0.63) | 0.057 |
| Sensitisation rate | 57.4% (27/47) | 39.1% (18/48) | 0.008 |
| nCor a 9 (SP) | |||
| sIgE levels | 0 (0–0.19) | 0 (0–0) | 0.038 |
| Sensitisation rate | 21.3% (10/47) | 10.9 (5/46) | 0.355 |
| Walnut | |||
| nJug r 3 (LTP) | |||
| sIgE levels | 0.88 (0.35–3.94) | 0.4 (0.12–1.13) | 0.058 |
| Sensitisation rate | 63% (17/27) | 81.6 (31/38) | 0.082 |
| rJug r 1 (SP) | |||
| sIgE levels | 0.32 (0–0.92) | 0 (0–0) | 0.001 |
| Sensitisation rate | 7.4% (2/27) | 50% (19/38) | <0.001 |
| nJug r 2 (SP) | |||
| sIgE levels | 0 (0–0) | 0 (0–0.07) | 0.125 |
| Sensitisation rate | 18.5% (5/27) | 21.6% (12/38) | 0.186 |
| Peanut | |||
| rAra h 9 (LTP) | |||
| sIgE levels | 0.57 (0.05–1.65) | 0.2 (0–0.63) | 0.045 |
| Sensitisation rate | 64.7% (22/34) | 36.8% (14/38) | 0.016 |
| rAra h 1 (SP) | |||
| sIgE levels | 0 (0–0) | 0 (0–0) | 0.602 |
| Sensitisation rate | 11.8% (4/34) | 13.2% (5/38) | 0.572 |
| rAra h 2 (SP) | |||
| sIgE levels | 0 (0–0.12) | 0 (0–0) | 0.003 |
| Sensitisation rate | 17.6% (6/34) | 5.3 (2/38) | 0.098 |
| rAra h 3 (SP) | |||
| sIgE levels | 0 (0–0) | 0 (0–0) | 0.573 |
| Sensitisation rate | 2.9% (1/34) | 2.6% (1/38) | 0.725 |
| nAra h 6 (SP) | |||
| sIgE levels | 0 (0–0.98) | 0 (0–0) | 0.004 |
| rate | 35.3% (12/34) | 13.2% (5/38) | 0.026 |
| Wheat | |||
| rTri a 14 | |||
| sIgE levels | 0 (0–0) | 0 (0–0.31) | 0.285 |
| Sensitisation rate | 0% (0/3) | 26.3% (33/126) | 0.143 |
Specific IgE (sIgE) levels (median values and interquartile range in brackets) and sensitisation rates to the LTPs and storage proteins tested for tolerant and allergic children. Children who had never eaten peach, peanut, walnut or hazelnut and those who had stopped eating the food several months to years before without evidence of allergic symptoms were excluded from the above-mentioned comparative analysis between allergic and tolerant individuals (19 cases were excluded for peach, 37 for hazelnut, 58 for peanut, and 65 for walnut).
Bold values show statistical significance (p value<0.05).
* The explanation that specific IgE is expressed as median values and interquartile range in brackets.
† Sensitization defined as specific-IgE levels >0.3 ISU by ISAC (Number of patients sensitized/Total number of allergic or tolerant individuals).
Peach allergic patients showed higher rPru p 3 sIgE levels by ImmunoCAP ISAC and ImmunoCAP than peach tolerant individuals (p=0.001). Walnut and hazelnut allergic children differed from tolerant ones in higher sIgE levels to the respective storage-proteins (rJug r 1, p=0.001; nCor a 9, p=0.038), but not in sIgE levels to the corresponding LTPs (nJug r 3/rCor a 8, p>0.05). In peanut-allergic children a greater difference was found in the sIgE levels to the respective storage-proteins (rArah 2, p=0.003; nAra h 6, p=0.004) than in the LTP (rAra h 9, p=0.045) (Table 3). No linear correlation was found between the number of plant-foods involved in allergic reactions and rPru p 3 sIgE levels by ImmunoCAP ISAC (Rho Spearman 0.066, p=0.458) or ImmunoCAP (Rho Spearman: 0.058, p=0.512).
Sensitisation to a particular plant-food LTP did not always cause clinical symptoms with that plant-food. Indeed, 69% (40/58) and 63% (17/27) of peach- and walnut-tolerant subjects had positive rPru p 3 and nJug r 3 sIgE, respectively. Similarly, 39.1% (18/46) hazelnut tolerant individuals had positive rCor a 8, whereas for peanut 36.8% (14/38, Ara h 9+) and for wheat 26.2% (33/126, rTri a 14+). Likewise, 60% (21/35) of children without a clinical history of nut allergy and 52.9% (9/17) of children with isolated stone-fruit allergy were sensitised to storage proteins.
26.2% (34/130) of the study population were sensitised to LTPs and not to any other plant-food panallergens included in the microarray. The main plant-foods involved in reactions among this subgroup of patients were: peach (n=23 allergic patients), peanut (n=8), apple (n=7), walnut (n=6). 64.7% (22/34) of these patients exclusively sensitised to LTP had experienced allergic reactions to nuts.
ROC curves for sIgE to LTPs and storage-proteins did not allow an accurate discrimination between tolerance and allergy to the respective plant-foods (peach, peanut, walnut, hazelnut), with AUC ranging from 0.531 to 0.720 (data not shown). ImmunoCAP did not improve microarray rPru p 3 sIgE diagnostic performance (AUC: 0.590). rPru p 3 sIgE by ImmunoCAP and by microarray showed very poor correlation (intra-class correlation coefficient: 0.322 (95% CI: 0.039–0.521, p=0.002). Children who had never eaten peach, peanut, walnut or hazelnut and those who had stopped eating the food several months to years before without evidence of allergic symptoms were excluded from the above-mentioned comparative analysis between allergic and tolerant individuals (14 cases were excluded for peach, 58 for hazelnut, 63 for walnut and 66 for peanut).
DiscussionTo our knowledge this is amongst the largest studies in children addressing molecular-based sensitisation in plant-food allergy, with special insight into LTPs. In agreement with previous studies on LTP sensitisation,11,21,25–28 symptoms severity was highly variable and a wide spectrum of plant-foods were involved in reactions. Accordingly, we have shown that LTP syndrome can occur from a very young age. However, in contrast to our previous study in adults from the same geographical area, sensitisation to other plant-food panallergens –especially storage proteins – was very common in our children. Interestingly, the clinical significance of LTP and/or storage protein sensitisation was unclear in a significant proportion of cases. Cross-reactivity might help explain this in some patients, for instance rCor a 8 or rAra h 9 sensitisation in children with stone-fruit allergy but tolerant to nuts. In addition to LTPs, 65% of cases were sensitised to storage proteins, which was associated with experiencing anaphylaxis and nut allergy. This finding raises the question whether LTP sensitisation is relevant in some patients, for instance in those exclusively allergic to nuts who are sensitised to storage proteins. Whether this likely silent LTP sensitisation might become clinically relevant over time and/or in the presence of cofactors, – or simply resolve – requires further prospective longitudinal studies. Our findings are concordant with a previous observation of frequent asymptomatic rPru p 3 sensitisation in an older cohort from Madrid, Spain, in which only 9% of patients had food allergy (median age: 27 years, range: 15–41).27 Likewise, 60% of patients without any nut or seed allergy were sensitised to storage proteins, whose clinical relevance in such a context is also unknown. Since asymptomatic sensitisation to LTP and/or storage protein was common (along with the fact that different allergenic components may explain clinical reactivity), the specific IgE testing by microarray failed to accurately discriminate allergic versus tolerant individuals to the plant-foods evaluated for the present study (i.e. peach, peanut, hazelnut, walnut). Similarly, a study in Italian children highlighted the limited value of specific IgE levels to foods other than Rosaceae to predict clinical allergy.17
With regards to reactions severity, specific IgE levels to LTPs did not correlate with reaction severity on history in our series, as previously described for peach allergy.17,29–31 Additionally, sensitisation to storage proteins was associated with anaphylaxis, suggesting that these allergens might involve higher potential for severe reactions than LTPs. In agreement with that, in an Italian adult series, storage-proteins sensitisation -rather than LTPs- was found more relevant for anaphylaxis and clinical reactivity to nuts.32,33 Nonetheless, in our study 31% of children exclusively sensitised to LTPs (and not to storage proteins) had experienced anaphylaxis (including anaphylaxis to nuts). Although this is a much lower proportion than that reported in our previous adult series (which was 75%), the potential of LTP to trigger anaphylaxis in children should not be underestimated.
Co-factors have been reported to enhance reactions severity in adults with LTP allergy.21,34,35 In our previous adult series co-factors were involved in reactions in 40% of cases. In contrast, we identified a cofactor (exercise) for reactions only in three children. We cannot exclude that this difference may be due to difficulties in evaluating cofactors in children, since parents might not report the coincidence of exercise (jumping, running) or intense emotions (crying, excitement), as these situations can be regarded as “normal life”. Further intervention studies would be required to determine the role of cofactors in LTP allergy in children, i.e., their ability to increase reactions severity and/or reduce patients’ reactivity threshold. Co-existent pollinosis has been reported to soften LTP-allergy symptoms.36,37 This was not reproduced in our study, since anaphylaxis rates were similar in children with or without sensitisation to pollen LTPs. Additionally, pollinosis and sensitisation to Art v 3 have been reported to contribute to LTP poly-sensitisation.16,17,31 In agreement with this, we observed that subjects sensitised to pollen LTPs had sIgE for a broader spectrum of plant-food LTPs than those who were not. Prospective studies are required to assess whether this phenomenon corresponds to a different primary sensitisation mechanism (i.e. via inhalation versus via oral/skin route) and to which extent sensitisation to pollen LTP contributes to “IgE spreading” or, alternatively, if it merely reflects broader cross-reactivity. We observed that sensitisation to rCor a 8 and rAra h 9 occurred almost exclusively in children sensitised to rPru p 3 and nJug r 3. These associations might suggest how sensitisations to the different plant-food LTPs develop overtime, which needs confirmation with prospective studies. Our observations are in agreement with two previous studies supporting Pru p 3 as the primary sensitiser in peach allergic adults with co-existent peanut38 and hazelnut allergy, respectively.39
In view of current evidence in the field of molecular-based diagnosis in plant-food allergy, it is difficult to determine to what extent these new techniques help the clinician in routine decision-making in terms of individual risk assessment or discrimination between allergy and tolerance.
Finally, we acknowledge several limitations in our study. First, it is a retrospective study, although we completed clinical data by reviewing all patients. Blinded food challenges -the gold standard for food allergy diagnosis- were not performed to confirm the diagnosis of plant-food allergy or to systematically determine allergy versus tolerance to other plant-foods.
In conclusion, plant-food allergy in the context of LTP sensitisation in children from Northeast Spain involves a diverse clinical picture (including anaphylaxis) and a broad range of triggers, as previously described in adults. However, co-sensitisation to storage proteins is common, which are associated to anaphylaxis and nut allergy. Sensitisation to LTP and/or storage proteins without clear clinical relevance is relatively common. Prospective studies are required to evaluate the role of these silent sensitisations in the future and/or when coinciding with cofactors. Caution is still required when interpreting the results of molecular-based diagnostic tools in clinical practice.
Ethical disclosuresPatients’ data protectionConfidentiality of data. The authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals for this investigation.
Conflict of interestThe authors declare that they do not have any potential conflict of interest. The study was self-funded.