Antibody deficiency comprises a heterogeneous group of disorders characterised by the body's inability to mount an effective antibody response to pathogens. Although it has been reported that asthma and allergic disease are frequent in antibody deficiencies, there are no data that evaluate and compare bronchial hyperreactivity (BHR) in all groups of antibody deficiencies. In this study, we aimed to evaluate and compare the frequency of BHR in patients with different antibody deficiencies.
MethodsThe study was carried out on 113 patients between ages 5 and 18 diagnosed with antibody deficiencies. The patients and their families were questioned on their history of asthma and allergic diseases. Allergic skin prick tests and non-specific bronchial provocation test with methacholine was done for all patients. Complete blood count and serum total IgE levels were measured.
ResultsThe mean age of the patients was 10.8±3.8 years and 66.4% were male. Within the study group 41.6% of the patients had selective IgA deficiency, 24.8% had IgG subclass deficiency, 14.2% had partial IgA deficiency, 10.6% had common variable immunodeficiency, 6.2% had transient hypogammaglobulinaemia and 2.7% X-linked agammaglobulinaemia. In total group, 42.5% had bronchial hyperreactivity with methacholine challenge test. BHR was more significant in both patients with selective IgA deficiency and partial IgA deficiency compared to those with IgG subclass deficiency (P=0.041 and P=0.038, respectively).
ConclusionBHR was high in antibody deficiencies, especially selective IgA deficiency compared to IgG subclass deficiency.
Antibody deficiency comprises a heterogeneous group of disorders characterised by the body's inability to mount an effective antibody response to pathogens. Antibody deficiency disorders may be congenital or they may develop later in life in response to environmental triggers or iatrogenic factors.1,2 It has been reported that asthma and allergic disease are frequent in antibody deficiencies.3–6
Asthma is an immunological disease that includes multiple inflammatory and clinical phenotypes, characterised by airway inflammation and bronchial hyperreactivity (BHR), and recurrent wheezing, cough, and shortness of breath.7 Airway hyperreactivity to various stimulant agents is a characteristic feature of bronchial asthma. Determination of airway hyperresponsiveness with methacholine challenge is a safe, simple, standardised, and reproducible diagnostic tool used in clinical practice to establish the presence of asthma.8
With regard to BHR in antibody deficiencies, two studies have been done; one being on selective IgA deficiency and the other on common variable immune deficiency.9,10 To our knowledge, there is no study that evaluates BHR in all groups of antibody deficiencies in the literature. In this study, we aimed to evaluate and compare the frequency of BHR in patients with different antibody deficiencies.
Materials and methodsStudy design and populationThis study was undertaken in Ankara Children's Health and Diseases Hematology-Oncology Hospital between June 2011 and January 2012, with the approval of the local ethics committee. The study was done on 113 patients between ages 5 and 18 diagnosed with antibody deficiencies. The patients and their families were questioned on their history of asthma and allergic diseases. Allergic skin prick tests and non-specific bronchial provocation test with methacholine was done for all patients. Since it can affect the methacholine challenge test, patients who developed bronchiectasis were excluded from the study. Complete blood count and serum total IgE levels were measured for all patients. A documented informed consent was taken from the patients and their parents.
Antibody deficienciesPatients were diagnosed and classified according to the clinical and laboratory criteria of PID reported by the IUIS Primary Immunodeficiency Diseases Classification Committee.11 Two types of IgA deficiency may be distinguished: selective IgA deficiency, with IgA level less than 6mg/dl, and partial IgA deficiency, with a level greater than 6mg/dl but less than 2 standard deviations below the age-adjusted mean level.
Allergy skin prick testsAllergy skin prick testing was performed for all of the patients for Dermatophagoides pteronyssinus, Dermatophagoides farinae, Cat and Dog dander, Alternaria, Cockroach, Aspergillus, Clodosporium, Betulaceae, Grass mix, Tree mix, Artemisia, Oleaceae, Saliceae, Parieteria, egg, wheat, peanut, hazelnut, milk, sesame, soya, fish, histamine, and negative controls (Stallergens, Antony, France). These tests were performed on the volar surface of both forearms, with results recorded after 15min. Results were considered positive when the mean wheal diameter was at least 3mm larger than that produced by the control.
Bronchial provocation testsMethacholine challenge was performed according to a protocol described by Cockcroft et al.12 as detailed previously.13 Briefly, after saline inhalation, doubling concentrations of methacholine were inhaled during a 2-min tidal breathing period every 5min, starting with 0.06mg/mL up to 8mg/mL or until a decrease in FEV1 of at least 20% (PC20) was obtained. The concentration of agents producing a PC20 was calculated by linear interpolation on the log dose–response curve. A PC20 of 8mg/mL or less was considered to represent a positive test result. Duplicate spirometry was performed at 0.5 and 1.5min after each inhalation. As suggested,14 methacholine was diluted in saline. The test was performed during the children's asymptomatic periods or at least four weeks after respiratory tract infection. On the challenge day, patients were queried about prior use of drugs, and none had used short- or long-acting agonists, antihistamines, or inhaled oral corticosteroids/leukotriene receptor antagonists for at least 24h, 1 week, and 3 months before the study, respectively.
Statistical analysisStatistical analysis was performed using the SPSS packet program. Values were either provided as numbers and percentages, or as mean±standard deviation, where applicable. Comparisons of the frequency of allergic diseases and other variables between patients with antibody deficiencies were made using the Chi-square test, Fisher's exact test and student's t-test. A P-value of ≤0.05 was considered indicative of statistical significance.
ResultsThe mean age of the patients was 10.8±3.8 years, and 66.4% were male. Within the study group 41.6% of the patients had selective IgA deficiency, 24.8% had IgG subclass deficiency, 14.2% had partial IgA deficiency, 10.6% had common variable immunodeficiency, 6.2% had transient hypogammaglobulinaemia and 2.7% X-linked agammaglobulinaemia.
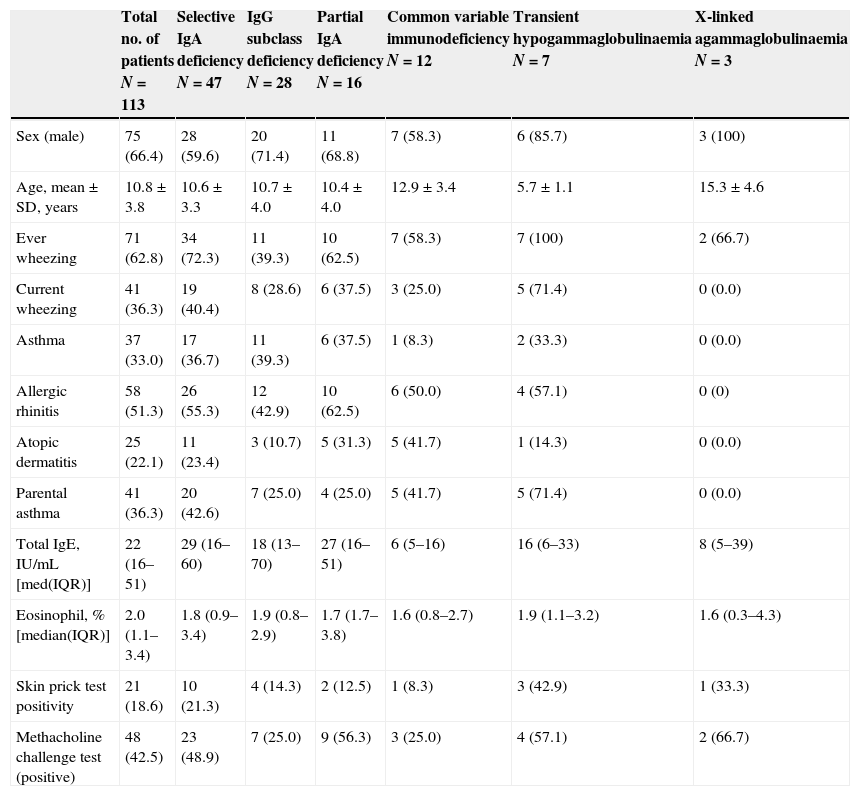
Allergic evaluation revealed that 62.8% of the patients had ever wheezing, 36.3% had current wheezing, 33% had physician-diagnosed asthma, 51.3% had a history of allergic rhinitis, 22.1% had a history of atopic dermatitis, 18.6% had a positive skin prick test and 42.5% had bronchial hyperreactivity with methacholine challenge test (Table 1).
Presence of allergic disease, bronchial hyperreactivity, laboratory findings and skin prick test positivity in patients with antibody deficiencies.
| Total no. of patients N=113 | Selective IgA deficiency N=47 | IgG subclass deficiency N=28 | Partial IgA deficiency N=16 | Common variable immunodeficiency N=12 | Transient hypogammaglobulinaemia N=7 | X-linked agammaglobulinaemia N=3 | |
|---|---|---|---|---|---|---|---|
| Sex (male) | 75 (66.4) | 28 (59.6) | 20 (71.4) | 11 (68.8) | 7 (58.3) | 6 (85.7) | 3 (100) |
| Age, mean±SD, years | 10.8±3.8 | 10.6±3.3 | 10.7±4.0 | 10.4±4.0 | 12.9±3.4 | 5.7±1.1 | 15.3±4.6 |
| Ever wheezing | 71 (62.8) | 34 (72.3) | 11 (39.3) | 10 (62.5) | 7 (58.3) | 7 (100) | 2 (66.7) |
| Current wheezing | 41 (36.3) | 19 (40.4) | 8 (28.6) | 6 (37.5) | 3 (25.0) | 5 (71.4) | 0 (0.0) |
| Asthma | 37 (33.0) | 17 (36.7) | 11 (39.3) | 6 (37.5) | 1 (8.3) | 2 (33.3) | 0 (0.0) |
| Allergic rhinitis | 58 (51.3) | 26 (55.3) | 12 (42.9) | 10 (62.5) | 6 (50.0) | 4 (57.1) | 0 (0) |
| Atopic dermatitis | 25 (22.1) | 11 (23.4) | 3 (10.7) | 5 (31.3) | 5 (41.7) | 1 (14.3) | 0 (0.0) |
| Parental asthma | 41 (36.3) | 20 (42.6) | 7 (25.0) | 4 (25.0) | 5 (41.7) | 5 (71.4) | 0 (0.0) |
| Total IgE, IU/mL [med(IQR)] | 22 (16–51) | 29 (16–60) | 18 (13–70) | 27 (16–51) | 6 (5–16) | 16 (6–33) | 8 (5–39) |
| Eosinophil, % [median(IQR)] | 2.0 (1.1–3.4) | 1.8 (0.9–3.4) | 1.9 (0.8–2.9) | 1.7 (1.7–3.8) | 1.6 (0.8–2.7) | 1.9 (1.1–3.2) | 1.6 (0.3–4.3) |
| Skin prick test positivity | 21 (18.6) | 10 (21.3) | 4 (14.3) | 2 (12.5) | 1 (8.3) | 3 (42.9) | 1 (33.3) |
| Methacholine challenge test (positive) | 48 (42.5) | 23 (48.9) | 7 (25.0) | 9 (56.3) | 3 (25.0) | 4 (57.1) | 2 (66.7) |
IQR, interquartile range.
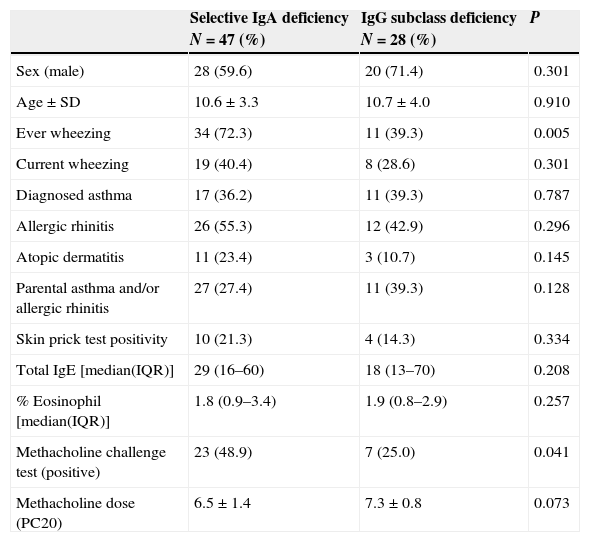
When patients with selective IgA deficiency and IgG subclass deficiency were compared, those with IgA deficiency had a higher rate of ever wheezing (P=0.005), whereas the rate of current wheezing was similar (P=0.301). Patients with partial IgA deficiency and IgG subclass deficiency were similar in those with IgA deficiency with a history of ever wheezing and current wheezing (P=0.138 and P=0.541, respectively). Besides, individual or familial history of other allergic diseases, mean total serum IgE level and total eosinophil counts did not differ between patients with selective IgA deficiency and IgG subclass deficiency (Table 2).
Comparison of selective IgA deficiency with IgG subclass deficiency.
| Selective IgA deficiency N=47 (%) | IgG subclass deficiency N=28 (%) | P | |
|---|---|---|---|
| Sex (male) | 28 (59.6) | 20 (71.4) | 0.301 |
| Age±SD | 10.6±3.3 | 10.7±4.0 | 0.910 |
| Ever wheezing | 34 (72.3) | 11 (39.3) | 0.005 |
| Current wheezing | 19 (40.4) | 8 (28.6) | 0.301 |
| Diagnosed asthma | 17 (36.2) | 11 (39.3) | 0.787 |
| Allergic rhinitis | 26 (55.3) | 12 (42.9) | 0.296 |
| Atopic dermatitis | 11 (23.4) | 3 (10.7) | 0.145 |
| Parental asthma and/or allergic rhinitis | 27 (27.4) | 11 (39.3) | 0.128 |
| Skin prick test positivity | 10 (21.3) | 4 (14.3) | 0.334 |
| Total IgE [median(IQR)] | 29 (16–60) | 18 (13–70) | 0.208 |
| % Eosinophil [median(IQR)] | 1.8 (0.9–3.4) | 1.9 (0.8–2.9) | 0.257 |
| Methacholine challenge test (positive) | 23 (48.9) | 7 (25.0) | 0.041 |
| Methacholine dose (PC20) | 6.5±1.4 | 7.3±0.8 | 0.073 |
IQR, interquartile range.
BHR was more significant in both patients with selective IgA deficiency as well as partial IgA deficiency compared to those with IgG subclass deficiency (P=0.041 and P=0.038, respectively). The concentration of methacholine producing a PC20 dose was lower in both the selective IgA deficiency and partial IgA deficiency groups than in the group with IgG subclass deficiency. However, this was not statistically significant (P=0.073 and P=0.235, respectively).
BHR in the selective IgA deficiency group was more significant in both those with ever wheezing and with current wheezing history compared to those without wheezing (P=0.030 and P<0.001, respectively). In the IgG subclass group there was no relation between BHR and presence of ever or current wheezing (P=0.249 and P=0.306, respectively).
Those with dust mite atopy among the group with selective IgA deficiency had a higher rate of BHR (P=0.033, not shown); however, there was no correlation between other allergens and BHR. There was no correlation between either dust mite or the other allergens and BHR in patients with IgG subclass deficiency. There was also no correlation between total IgE and BHR (P=0.750).
When patients with CVID and those with IgA deficiency (selective or partial) were compared, apart from physician-diagnosed asthma being more significant in IgA deficiency, individual or familial history of allergic diseases, mean total serum IgE level, total eosinophil counts, skin prick test results and BHR were similar.
DiscussionThe frequency of BHR and allergic disease was evaluated in patients with antibody deficiency in this study. Approximately two-thirds had a history of ever wheezing, one-third had a history of current wheezing, one-third had physician-diagnosed asthma, one half had a history of allergic rhinitis, one-fifth had a history of atopic dermatitis and almost half had BHR and one-fifth had a positive skin prick test. Although recurrent respiratory tract infections would result in congestion in the upper respiratory tract causing confusion with wheezing, the frequency of the allergic disease symptoms in our patients was apparently higher than that in the general population according to previous studies in our country.15,16
In previous studies it has been reported that the frequency of atopy and allergic disease has increased in patients with selective IgA deficiency.3,17–19 Furthermore, it has been reported that IgG subclass deficiency is high in severe asthma patients. However, this group of patients is on high steroids so it has been thought that the IgG subclass deficiency would be as an effect of steroid treatment. Correspondingly, it has been reported that IgG subclass levels in non-selected asthmatic patients are not different from the general population.20 In our study, ever wheezing history and BHR were more prevalent in patients with selective IgA deficiency than in patients with IgG subclass deficiency. This observation supports the concept that the absence of the protective effect of secretory IgA on the mucosal surface allows the passage of aeroallergens in the respiratory tract, resulting in increased incidence of allergic diseases associated with selective IgA deficiency by increasing the likelihood of sensitisation against such antigens.21
Papadopoulou et al. reported that patients with selective IgA deficiency had frequent BHR in those with a history of current wheezing, whereas there was no correlation between ever wheezing history and BHR.9 In our study in the selective IgA deficiency group, BHR was more significant in both patients with a history of current wheezing or ever wheezing, whereas there was no correlation between BHR and wheezing (current or ever) in the IgG subclass group. These findings suggest that in patients with lack of secretory IgA, symptoms of asthma are more valuable in diagnosing asthma than in patients with the other immunoglobulin deficiencies.
It is a known fact that house dust mites are an important environmental risk factor for BHR and asthma.22–24 In a study by Papadopoulou et al., among the patients with aeroallergen sensitivity, patients with selective IgA deficiency had a higher BHR frequency compared to patients without selective IgA deficiency, and this is thought to be due to a further increase in BHR as a result of recurrent infections.9 In our study, the selective IgA deficiency group had more significant BHR in those with house dust mite sensitivity. However, there was no correlation between BHR and house dust mite sensitivity in the IgG subclass group. These findings suggest that increased frequency of BHR in patients with selective IgA deficiency seems to be related to decreased mucosal protection against allergens due to lack of secretory IgA rather than recurrent respiratory tract infections.
Nagao et al. reported that mucosal secretory IgA was absent in selective IgA deficiency and low in partial IgA deficiency.25 Plebani et al. reported that atopic disease and total IgE levels were similar in patients with selective IgA deficiency and patients with partial IgA deficiency.26 Similar to these findings, we also found that patients with selective IgA deficiency and patients with partial IgA deficiency had similar individual or familial atopy histories, total IgE levels, skin prick test positivity and BHR. For this reason we thought that the total absence of secretory IgA is not a prerequisite for the malfunctioning of the defensive mechanism of the mucosa against allergens but that even low levels of IgA can cause this.
It has been reported that patients with CVID have similar mucosal immunodeficiency to that in IgA deficiency that eases the occurrence of BHR and asthma.10 In our study even though there were similarities in individual and familial atopy, skin prick test positivity and BHR between patients with CVID and patients with IgA deficiency, physician-diagnosed asthma was less significant in patients with CVID compared to patients with IgA deficiency. This may be related to the high rate of recurrent infections in patients with CVID which can mask asthma and cause a delay in its diagnosis.
It is known that in the absence of B lymphocytes and IgE, cytokines produced by T lymphocytes can induce respiratory tract inflammation and airway hyperresponsiveness.27 This could explain the allergic reactions in XLA patients with lack of B lymphocytes. However, it has been reported that T helper1 (Th1) activity is high and T helper2 (Th2) activity is low in XLA patients compared to healthy controls.28 Shabestari et al. reported a seven-year-old XLA patient with asthma and aeroallergen and food sensitisation.29 In our study two of the three XLA patients had an ever wheezing history, two had BHR and one had a positive skin prick test. For this reason we thought that Th2 may be high in some XLA patients.
In conclusion, bronchial hyperreactivity and allergic disease symptoms seem to be high in antibody deficiencies – especially selective IgA deficiency. For this reason, patients with antibody deficiencies, especially those with recurrent respiratory symptoms, should be evaluated for asthma and allergic disease with bronchial hyperreactivity being more significant in selective IgA deficiency compared to IgG subclass deficiency.
Ethical disclosuresProtection of human subjects and animals in researchWe declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Patients’ data protectionWe declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients mentioned in the article. The author for correspondence is in possession of this document.
Conflict of interestAll of the authors have no conflicts of interest in the manuscript.