Medical gas hydrogen (H2) has a special role in airway inflammation; however, the effect of H2 on allergic rhinitis (AR) remains unclear. This study explored the possible roles of H2 on the pathogenesis of AR and observed the influences of H2 on cytokines IL-4 and IL-13.
MethodsAn AR guinea pig model was established by nasal ovalbumin sensitisation. Eighteen guinea pigs were divided into three groups, namely, saline control, AR-sensitised, and hydrogen-rich saline (HRS)-treated groups, with each group having six guinea pigs. The frequencies of sneezing and scratching were recorded. The IgE level and cytokine (IL-4 and IL-13) levels in the serum were measured. The expression levels of IL-4 and IL-13 mRNA and protein in the nasal mucosa were also determined by real-time reverse transcriptase–polymerase chain reaction and Western blot. We also observed the infiltration of cytokine (IL-4 and IL-13) in nasal mucosa by immunofluorescence.
ResultsThe frequencies of sneezing and scratching, as well as the levels of IgE, IL-4, and IL-13, in the serum were higher in the AR group than in the control group (p<0.01), whereas all these parameters were decreased significantly after HRS treatment (p<0.05). The expression levels of IL-4 and IL-13 mRNA and protein in the nasal mucosa were also lower in guinea pigs treated with HRS than those in the AR group (p<0.05).
ConclusionsHRS could affect anti-inflammation in AR and decreased the expression of IL-4 and IL-13.
Rhinitis, especially allergic rhinitis (AR), is a major health problem. Several treatments are available for this condition, but none is ideal. Recent studies have shown that gas signal messengers, such as nitric oxide (NO), carbon monoxide (CO), and hydrogen sulphide (H2S), play important regulatory roles in allergy medicine, which is a rapidly emerging field.1,2
Molecular hydrogen as an antioxidant possesses protective and therapeutic values because of its ability to selectively reduce cytotoxic reactive oxygen species (ROS). In 2007, Ohsawa et al.3 reported that 2% hydrogen can effectively remove free radicals and evidently reduce cerebral ischaemia–reperfusion (I/R) injury. They found that the treatment effect of hydrogen on cerebral I/R injury is attributed to its ability to diffuse rapidly across membranes, and hydrogen could reach and react with cytotoxic ROS and thus protect against oxidative damage in inflammation. Signal messengers, such as NO, CO, H2S, and hydrogen (H2), are also considered to play an important role in the inflammatory processes of the upper and lower respiratory tract physiology.4,5 However, the role of H2 in AR has neither been elucidated nor reported in previous studies.
AR is a chronic inflammatory disorder of the nasal airways with Th1/Th2 imbalance. IL-4 and IL-13 are immunoregulatory cytokines that are predominantly secreted by activated Th2 cells. Over the past several years, IL4 and IL-13 have been generally acknowledged as key mediators in the pathogenesis of AR, and they directly influence Th2 cell maturation and drift. IL-4 and IL-13 share the same receptor subunit and play pivotal roles in IgE-dependent inflammatory reactions, as well as acting on B cells to induce IgE production. IL-13 is implicated in various allergic responses, including airway hypersensitivity, mucus hypersecretion, AR, and asthma.6,7
To clarify the role of H2 in the pathogenesis of AR, we used hydrogen-rich saline (HRS) instead of H2 for the treatment of guinea pigs because H2 cannot be controlled. The clinical symptoms of animals, including sneezing and nose rubbing, and IgE were studied as inflammation markers. The expression levels of IL-4 and IL-13 in the serum and nasal mucosa were also studied by ELISA, real-time reverse transcriptase–polymerase chain reaction (RT–PCR), and Western blot to reveal the influence of H2 on Th2 cytokine in AR.
MethodsMaterial and animal modelsHigh-purity H2 gas was dissolved in saline for 6h at 0.4MPa to reach a supersaturated level, and gas chromatography was conducted to measure the actual concentration of hydrogen saline.8 The HRS was stored at atmospheric pressure and 4°C in an aluminium bag with no dead volume. HRS was freshly prepared every week to ensure that the concentration was always higher than 0.6mmol/L. Gas chromatography (Biogas Analyzer Systems-1000; Mitleben, Japan) was performed to confirm the hydrogen content in water.
Mature healthy male guinea pigs weighing ±230g were purchased from National Rodent Laboratory Animal Resources (Shanghai, China). All animal care and experimental procedures were in accordance with the NIH guidelines and were approved by the Tongji University Institution Animal Care and Use Committee.
The AR models were prepared as follows. Eighteen guinea pigs were randomly divided into three groups (n=6 each group), namely, AR-sensitised, HRS-treated, and control groups. The AR-sensitised and HRS-treated groups were sensitised with ovalbumin (OVA). Each guinea pig was first sensitised intraperitoneally with 0.3mg of OVA and 30 of mg AL(OH)3 every other day for a total of seven times. From day 15, the guinea pigs were treated with 0.5% OVA aerosol to stimulate AR symptoms for five times. Subsequently, each side of the nasal cavity was given 20L of 2% OVA solution intranasally once a day to maintain AR symptoms.
From day 20, the guinea pigs of the HRS-treated group were given HRS at a dose of 10mL/kg as well as intraperitoneally injected and were also given 20μL of HRS to each side of the nasal cavity intranasally once a day. The guinea pigs in the AR-sensitised group were given the same dose of saline every day. The treatment continued for 14 days. The control group was also given the same dose of saline treatment daily.
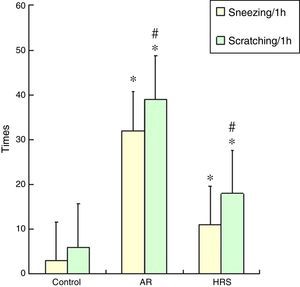
Observation of frequencies of scratching and sneezingThe frequencies of scratching and sneezing were assessed using the procedure previously described by Al Suleimani but with modifications.9 The scratching and sneezing instances were counted for 1h directly following a nasal challenge. Sneezing was characterised by an explosive expiration just after deep inspiration. Scratching was characterised by an external perinasal scratch with the animal's forelimbs.
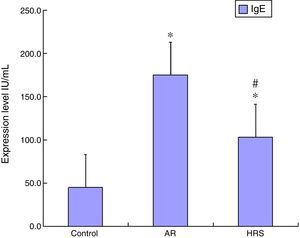
Determination of IgE and cytokines IL-4 and IL-13 in serumThe guinea pigs were anaesthetised by intraperitoneal administration of pentobarbital (40mg/kg). These animals were sacrificed by rapid decapitation, and then blood and nasal mucosa were collected. Biopsies of the nasal mucosa were taken from the inferior turbinate and immediately placed in liquid nitrogen. The levels of IgE, IL-4, and IL-13 of the guinea pigs were determined by ELISA (RB Inc., Maryland, USA).
RNA isolation and RT-PCR for IL-4 and IL-13RT-PCR was performed to determine the expression levels of IL-4 and IL-13 mRNAs in nasal mucosa. The total RNA was extracted using Trizol™ reagent (Invitrogen Inc., MD) following the manufacturer's instructions. Subsequently, cDNA was synthesised using a cDNA synthesis kit (PrimeScript RTase, TaKaRa Inc., Japan). To determine the expression of IL-4 and IL-13, we performed a fluorescent quantitative real-time RT-PCR assay. The following primers were used: IL-4 (forward): 5¿-GCAAGAAATCATCCAGCACCTC-3¿ (213bp), (reverse): 5¿-TGTCTCAGGA CGCTGGGGT-3¿ (213bp); IL-13 (forward): 5¿-TGTC TTCCACCGTAGCCGT-3¿ (203bp), (reverse): 5¿-GCTCAGTATCTTCTGGGT CCTC-3¿ (203bp); and GAPDH (forward): 5¿-AAAGGCATCTTGGGCTACACC-3¿ (153bp), (reverse): 5¿-GCTGTA GCCGAACTCATT GTCATA-3¿ (153bp). For each sample, the amounts of both target and endogenous control (GAPDH) were determined.
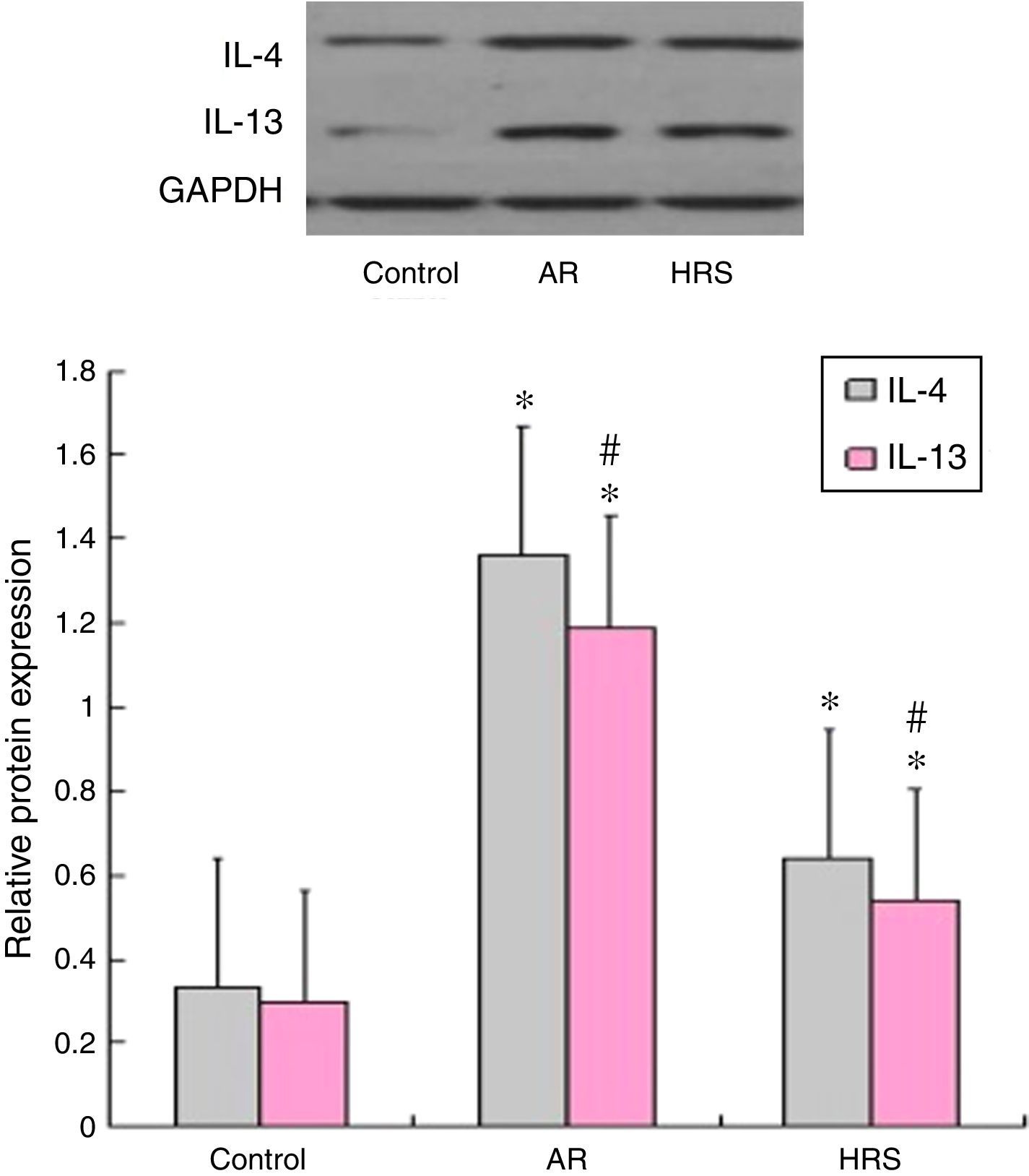
Western blot analyses of IL-4 and IL-13The guinea pig nasal mucosa (10mg), which was frozen in liquid nitrogen, was homogenised in 1mL of protein lysis buffer (PBS containing 0.1% Triton X-100) and centrifuged at 14,000g for 10min at 4°C. Nasal mucosa lysates (50g) from each group were analysed by Western blot. The blots were blocked with PBS-T containing 1% skim milk and then incubated with a 1:1000 dilution of anti-IL-4 or a 1:1000 dilution of anti-IL-13 antibody at room temperature. After three additional washes, the blots were incubated with anti-mouse secondary antibody (1:5000) then conjugated to horseradish peroxidase for 1h at room temperature. The bands were visualised using EZ-ECL detection reagents. The second batch of images was quantified using Quantity One software. GAPDH was employed as an endogenous control for protein normalisation. The experiments were performed in duplicate.
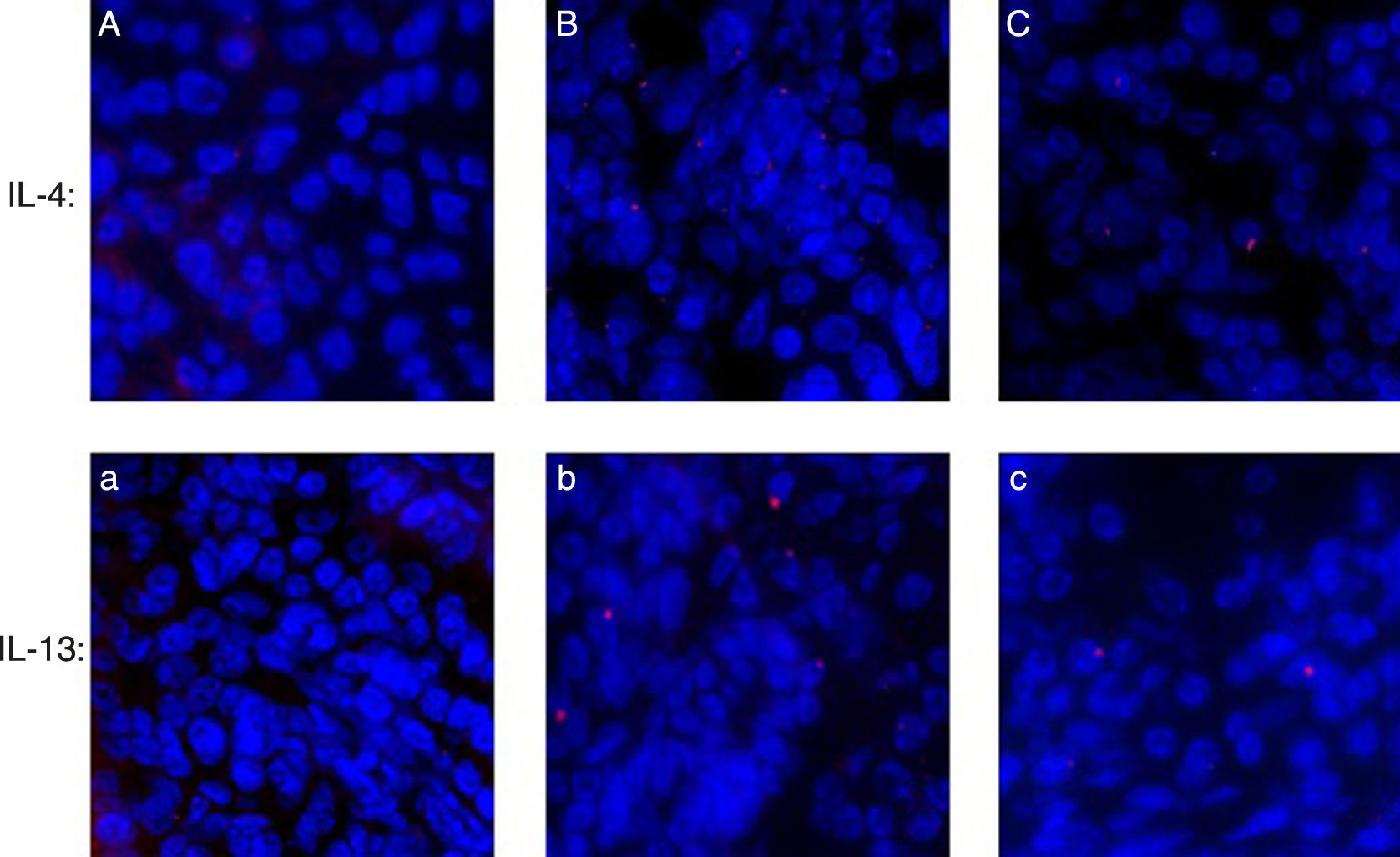
Immunofluorescence of IL-4 and IL-13 in nasal mucosaTissue from the specimens were fixed in 10% buffered formalin. Immunohistochemical stain were performed on formalin-fixed and paraffin-embedded 4 um sections. The tissue sections were deparaffined, and permeabilised in precooled acetone at −20°C for 15min. Slides were washed in PBS. Normal goat serum (10% in PBS) was used to block non-specific antibody binding. The slides were incubated for 1h at room temperature with anti-IL-4 rat monoclonal antibody (1:100, Boster Co. BA0980-1, Ltd., China). Slides were washed twice in PBS, incubated for 30min at room temperature with goat anti-rabbit Cy3-conjugated IgG (1:50, Boster Co. BA1032 Ltd., China). Slides were washed twice in PBS and once in distilled water and allowed to dry. Fluorescence microscope was applied to observation and photograph. The method of IL-13 was the same as IL-4.
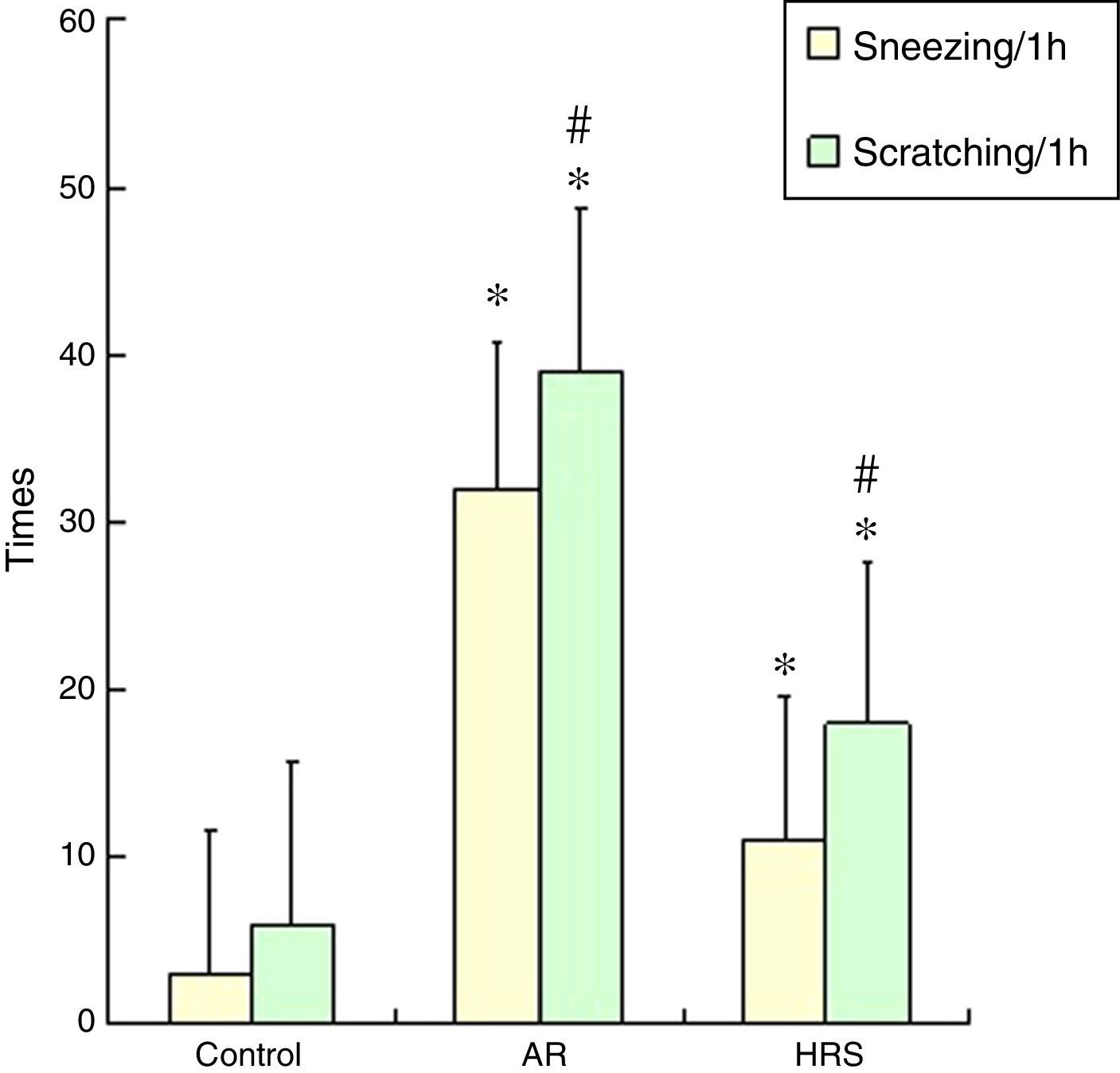
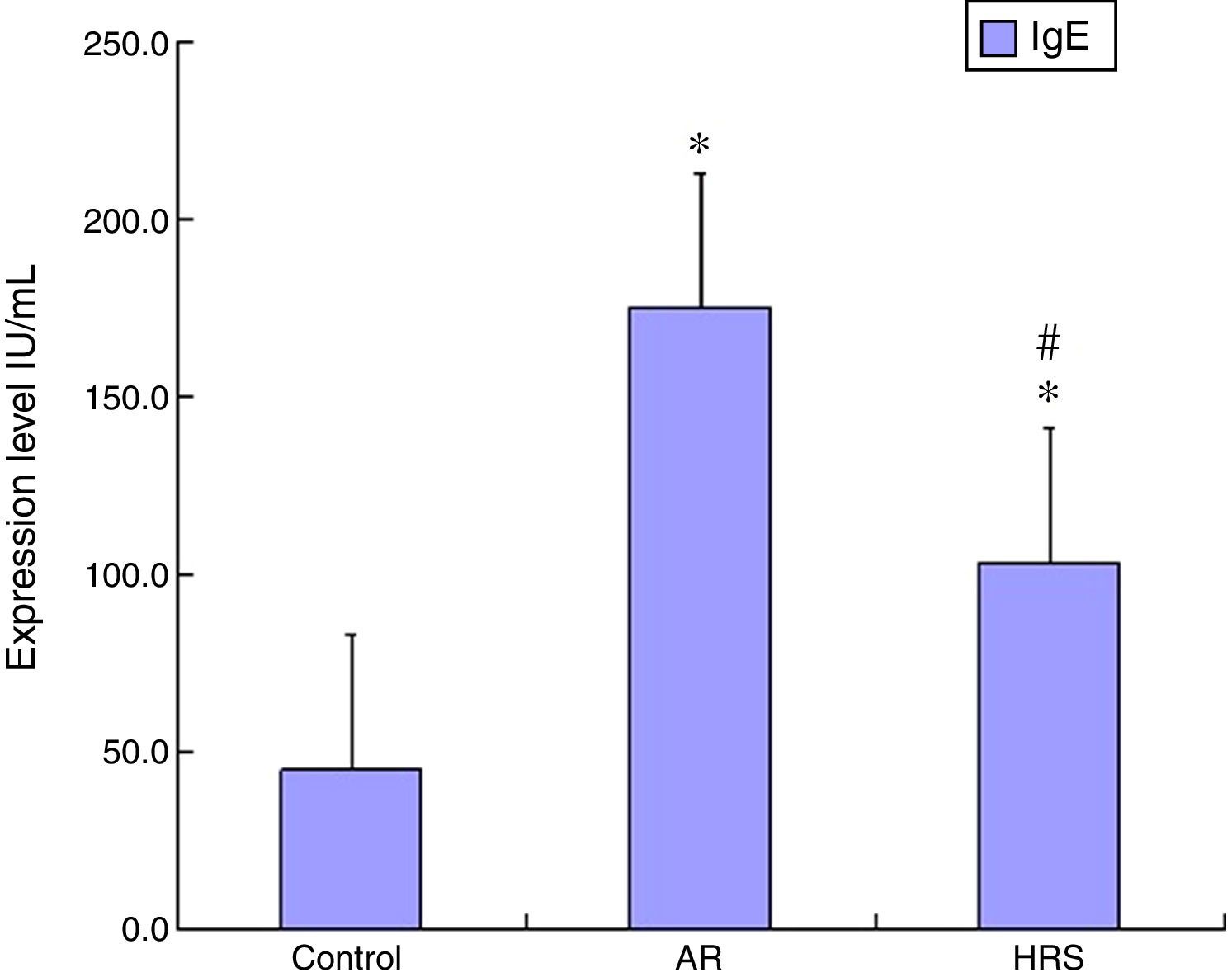
ResultsFrequencies of scratching and sneezing and IgE level in serumThe frequencies of scratching and sneezing are shown in Fig. 1. The frequencies of scratching and sneezing in the AR-sensitised group were significantly increased compared with the control group (p<0.05). In the HRS-treated group, the frequencies of scratching and sneezing decreased significantly compared with those of the AR-sensitised group (p<0.05). The level of IgE in the AR-sensitised group was higher than that in the control group (p<0.01). After HRS treatment, the IgE content in the guinea pigs was significantly decreased (p<0.01) (Fig. 2). All these results indicate that hydrogen could reduce the inflammatory response and IgE level of AR.
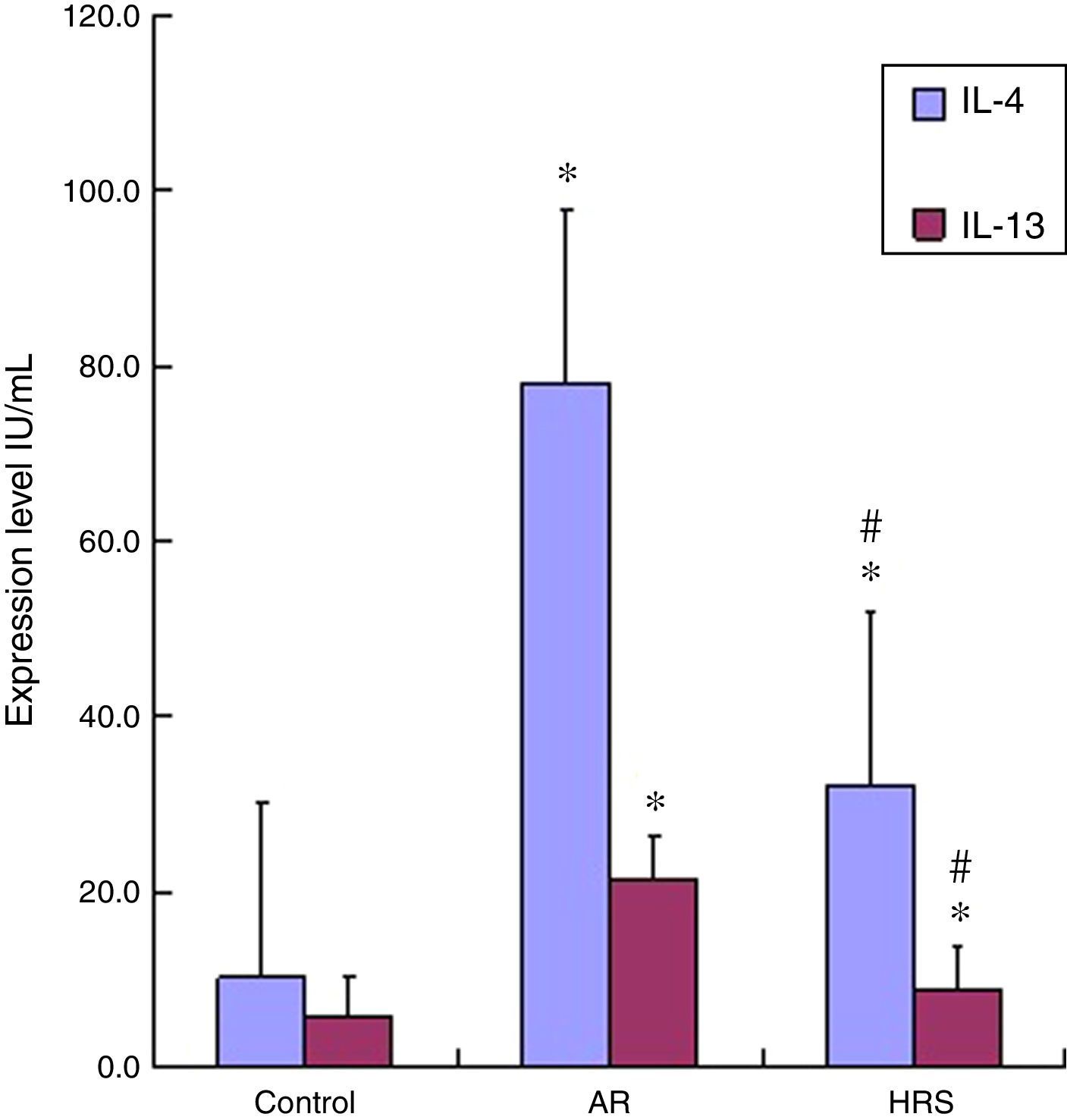
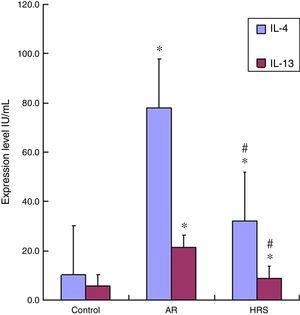
The IL-4 and IL-13 levels in the serum of the AR-sensitised group were higher than those of the control group (p<0.01 for each cytokine). The IL-4 and IL-13 levels decreased significantly after guinea pigs were treated with HRS compared with those of the AR group (p<0.01 for each cytokine) (Fig. 3). Therefore, hydrogen could decrease IL-4 and IL-13 levels in AR.
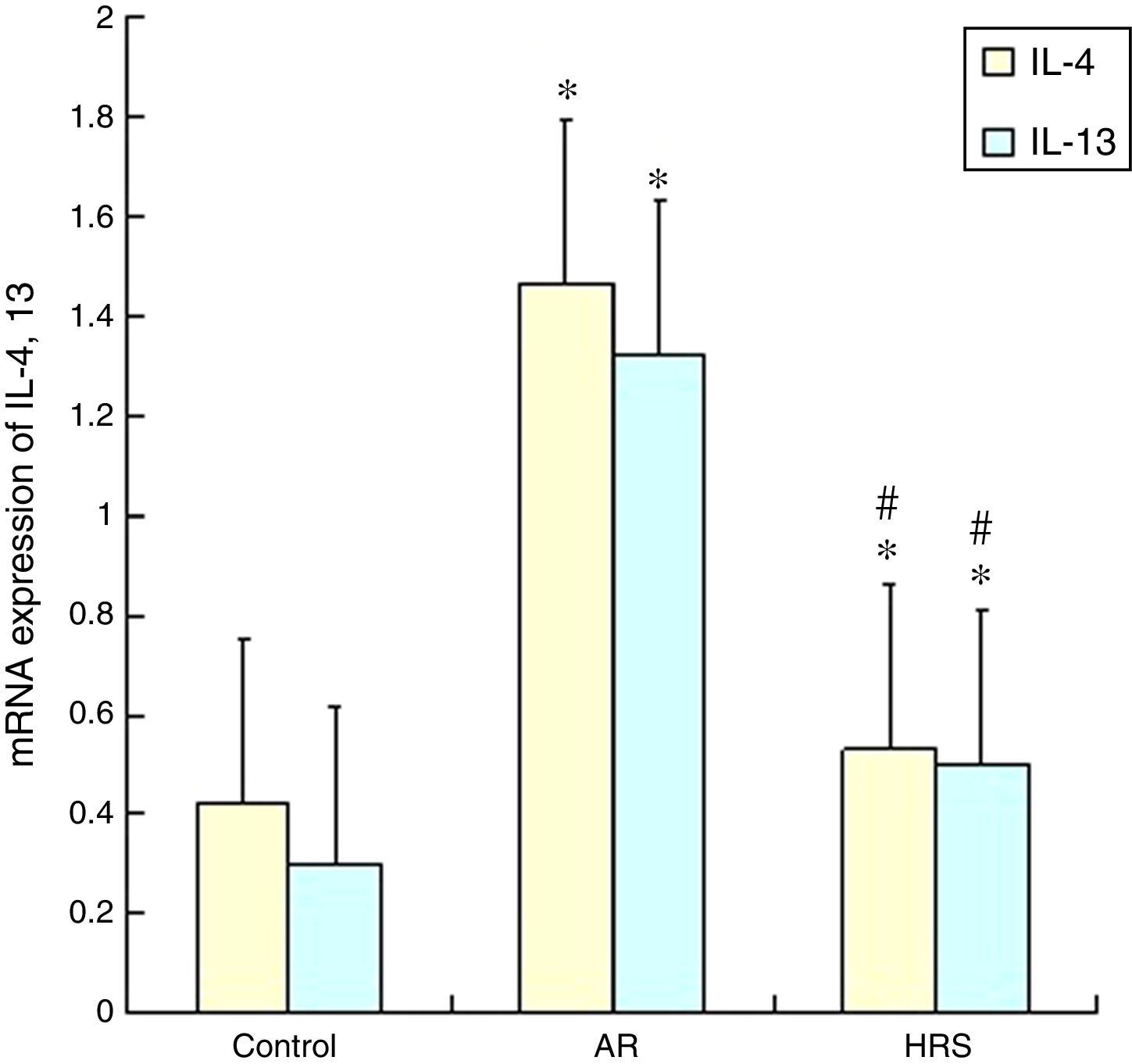
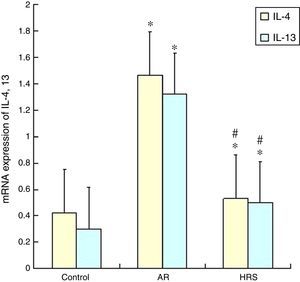
Reduction of IL-4 and IL-13 mRNA expression in nasal mucosa by HRS treatmentThe IL-4 and 13 mRNA expression in the nasal mucosa of the AR-sensitised was significantly increased (p<0.05). However, the mRNA expression of IL-4 and IL-13 of the HRS-treated group decreased significantly compared with that of the AR-sensitised group (p<0.05 for each). These results indicate that hydrogen could suppress the IL-4 and IL-13 mRNA expression in allergic inflammation conditions (Fig. 4).
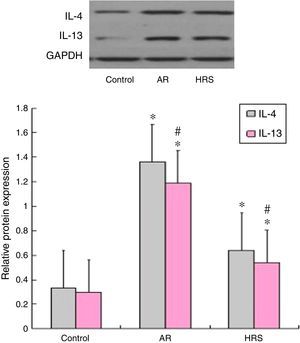
Western blot results of IL-4 and IL-13 expressionAs shown in Fig. 5, the Western blot analysis indicated that the expression levels of the IL-4 and IL-13 proteins in the AR-sensitised group were higher than those in the control groups (p<0.05 for each cytokine). After HRS treatment, the corresponding protein levels of IL-4 and IL-13 in the nasal mucosa were decreased significantly compared with those of the AR-sensitised group (p<0.05 for each cytokine). These results suggest that hydrogen not only suppressed the expression of IL-4 and IL-13 mRNA but also suppressed the protein in the nasal mucosa of the AR-sensitised models.
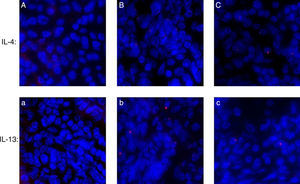
Assessing IL-4 and IL-13 expression by immunofluorescenceAs shown in Fig. 6, IL-4 and IL-13 expressed in the AR-sensitised group more than control groups; and after HRS treatment, the level of IL-4 and IL-13 were decreased significantly in the HRS group.
DiscussionThe current study is the first to show the anti-inflammation effect of hydrogen on AR-sensitised guinea pigs. Previous studies showed that gaseous transmitters, such as NO, CO, and H2S, play important roles in the pathogenesis of AR.10,11 Hydrogen, which is a colourless gas with the lightest element, is increasingly recognised as a new member of the growing family of “gasotransmitters”. In 2007, researchers from Japan reported that hydrogen gas possesses antioxidant and anti-apoptotic properties that protect the brain against I/R injury and stroke by selectively reducing hydroxyl radicals OH and ONOO− in cell-free systems.1 Through an in vitro study, the researchers demonstrated that hydrogen functions as a scavenger of OH and put forward the therapeutic value of hydrogen in many disease models.
More recently, several reports have shown that hydrogen gas and HRS could effectively inhibit inflammation and oxidative stress.12–15 The value of hydrogen therapy in cases of inflammation of the respiratory system has been reported. In a rat model of lung injury induced by intestinal I/R, HRS treatment, compared with saline treatment, decreased neutrophil infiltration, lipid-membrane peroxidation, and levels of the pro-inflammatory cytokines IL-1 and TNF in lung tissues, thereby attenuating lung injury.13 In a previous allergic inflammation research, HRS reduces airway inflammation by inactivating NF-κB in a murine model of asthma.16 The role of oxidative stress is well appreciated in the development of AR. Oxidative stress in AR is speculated to be initiated by the products of activated lymphocytes and infiltrating eosinophils and neutrophils, which propagate rapidly to epithelial and endothelial cells, leading to tissue damage and organ dysfunction. Our research provided evidence that HRS could attenuate OVA-induced AR symptoms, such as nasal scratching and sneezing. Furthermore, the results of the current study demonstrated that the IgE level decreased after HRS treatment and that HRS had anti-oxidative effects and alleviated the symptoms of AR.
The pathophysiology of AR is chronic airway inflammation, with infiltration of eosinophils, mast cells, and CD4+ T lymphocytes that express Th2 cytokines, such as IL-4, IL-5, and IL-13.17,18 Lymphocytes develop into either Th1 or Th2 cells. They can be classified based on their cytokine production profile in the immune system; Th1 cells secrete IFN-γ and TGF-β, which induce macrophages and killer T cells for cellular immunity or delayed hypersensitivity, mostly in bacterial and viral infections.19 Th2 cells produce IL-3, IL-4, IL-10, and other cytokines that are mainly involved in IgE-mediated delayed type-1 hypersensitivity reactions and are facilitative of the recruitment of eosinophils.20 IL-4 and IL-13 are immunoregulatory cytokines predominantly secreted by activated Th2 cells and key mediators in the pathogenesis of allergic inflammation, such as AR and asthma. IL-13 shares with IL-4 the same receptor subunit, and they respond by signalling through the T-cell antigen receptor and mast cells. IL-13 responds through eosinophils and basophils when a cross-linkage of the high-affinity IgE receptor occurs. IL-4 and IL-13 play a pivotal role in IgE-dependent inflammatory reactions and act on B cells to produce IgE. They are implicated in various allergic responses, including airway hypersensitivity and mucus hypersecretion. Therefore, a change in the IL-4 and IL-13 expression is the critical response of AR inflammation.
To explore the effect of HRS on inflammation in AR associated with changes in IL-4 and IL-13, we used ELIS, RT–PCR, and Western blot to analyse the effects of HRS on IL-4 and IL-13 expression. In the experiment, the IL-4 and 13 levels were successfully down-regulated in the serum of the HRS-treated group compared with those in the serum of the AR-sensitised group. The HRS treatment also alleviated the symptoms of AR. The results also showed that the expression levels of IL-4 and IL-13 mRNA and protein in the nasal mucosa were decreased significantly in the HRS-treated group compared with those in the AR-sensitised group. Therefore, HRS can suppress the production of cytokines by inhibiting IL-4 and IL-13 gene expression and consequently alleviate the symptoms of AR. This phenomenon is accompanied by suppression of IgE level, leucocyte adhesion, leucocyte infiltration, and disappearance of oedema. All these results suggest that HRS could reduce inflammation probably by suppressing the expression of IL-4 and IL-13 in the guinea pigs model of AR.
ConclusionsThe results demonstrated that HRS could attenuate AR inflammation by possibly inhibiting the expression of IL-4 and IL-13. However, additional investigations should be conducted to identify the underlying mechanisms of the signalling pathways involved in HRS anti-AR inflammation in the future.
Ethical disclosuresConfidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Conflict of interestThe authors declare no conflict of interest in the publication of this paper.
This work was supported by the Natural Science Foundation of Tongji Hospital (JYB1302).