Chronic idiopathic urticaria (CIU) is characterized by multiple swollen raised areas on the skin that are intensely itchy. These lesions can last less than 24 hours and persist for more than 6weeks.1–3 CIU affects 0.5-1 % of the population and causes a significant decrease in the quality of life.
The major advance in our understanding of CIU in recent years has been the discovery that in 30–50 % of patients with CIU the disease is due to an autoimmune process, and therefore is not strictly ‘idiopathic.’1
Patients with chronic autoimmune urticaria (CAU) have circulating antibodies against the high affinity receptor FceRI or against IgE.4,5 These antibodies are functionally active and therefore they stimulate the release of histamine by basophils and mast cells. The existence of these antibodies has been confirmed by the use of Western Blot and ELISA.6 These antibodies can also be identified with the autologous serum skin test due to the ability of serum samples to release histamine from basophils. There is an important correlation between a positive autologous serum skin test and the disease activity.2 CIU causes an important decline in the quality of life and increases annual direct and indirect health care costs.7,8
There are few studies that reveal the presence of CAU in children even though in some series the prevalence of CAU in children ranges from 31 % to 40 %.9 In developing countries this prevalence is unknown.
Patients with CAU are poor responders to antihistamine therapy, and immunosuppressive therapy is warranted.10,11 Management of children with CAU in third world countries is very limited due to the unavailability of in vitro testing; the lack of access to specialised centres; and the high cost of medicines. These conditions are a real challenge for the allergist and the dermatologist.
IntroductionUrticaria can be classified on the basis of its duration, frequency and causes. Depending on its duration urticaria can be classified as acute or chronic. Acute urticaria can be defined as the presence of hives for less than six weeks. Chronic urticaria (CU) is characterized by the persistence of the symptoms for more than 6weeks.12 Around 40 to 50 % of patients with CIU demonstrate an immediate wheal and flare response to intra-dermally injected autologous serum.13 This led to the concept of CAU, Gruber et al5 detected the presence of antibodies against IgE in different types of urticaria and they were the first to propose that these antibodies could have been implicated in the development of the skin lesions. it was later demonstrated that these antibodies stimulated the release of histamine and reacted against IgE and its receptor.4 The reason why these patients produce autoantibodies is unknown.14–16
The majority of studies have been carried out in adults and thus there is little information about the etiology of CAU in children.17–19 Physical urticaria appears to be the most frequent cause of CU in children.11,20 The search for CAU using the autologous serum skin test and by the demonstration of functionally active autoantibodies is usually not performed.21
Brunetti et al confirmed that CAU could explain as much as 30 % of CU in children and that searching for this disease could drastically decrease the percentage of CIU from 52 % to 20 %.9
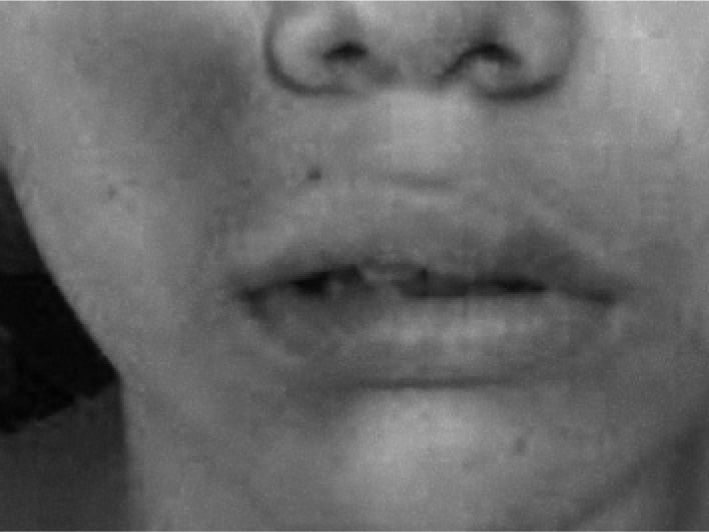
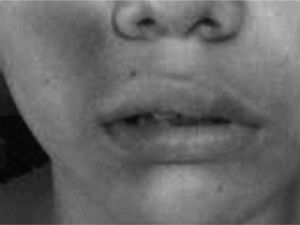
Clinical CaseThirteen year old patient with a two year history of urticaria accompanied by headaches and angio-oedema. The hives appeared in different parts of the body, they were itchy, variable in size and lasted less than 24 hours. They appeared more frequently at night (figs. 1 and 2).
The angio-oedema was non-pitting and was accompanied by severe headaches and a sensation of oppression in the larynx. In the last months the episodes had increased in frequency and intensity, appearing even during the day and importantly affecting the patient's quality of life to the point where he was unable to attend school. Exacerbating factors include stress, swimming, NSAIDS, chocolate and artificial juices. Familial history included allergy and autoimmune disorders. Gammaglobulin, thyroid hormones, antithyroid antibodies and complement levels were normal. A hepatitis panel and ANA were also negative. The Prick test was negative for allergens. Stool examination was negative for parasites. A chest x-ray did not reveal any abnormality. The autologous serum skin test was positive on three occasions performed on three different days.
PathogenesisThe pathogenesis of CAU is probably related with circulating mediators which stimulate the release of histamine from dermic mast cells but not from basophils.22,23
This mast cell histamine releasing factor has a molecular weight > 30kDa and has been partially characterized.24 There are other studies on a cytokine produced by mononuclear cells in the peripheral blood that could increase the release of histamine by basophils, eosinophils and T cells.
The anomalies found in some patients with CAU could have a cellular or a humoral origin which opens new interesting research possibilities, especially after the report of an aberrant regulation of the p21 Ras via in the mononuclear cells of patients with CAU.25,26
The autoantibodies against the high affinity receptor may be not functional and be present in certain autoimmune disorders such as dermatomiositis and pemphigus vulgaris. These autoantibodies react against IgG2 and IgG4, in contrast with the antibodies found in CAU, which release histamine and react primary against IgG1 and IgG3.27
The circulating IgG isotypes responsible in the pathogenesis of CAU are IgG3 (primary), IgG1 (frequent) and IgG4 (occasional).2 The binding of the autoantibodies to FceRIa may be competitive or non-competitive.8 This binding leads to the activation of the classical complement pathway releasing C5a.8 C5a binds to the respective receptors on the mast cell surface augmenting histamine secretion by degranulation.28,29
CAU is an autoimmune disorder because the autologous serum skin test can be reproduced by passively transferring the affected individual's serum into the skin of healthy individuals. An important correlation exists between serum levels of functionally active autoantibodies and the severity and prognosis of the disease.30,31
Clinical manifestationsPatients with CAU present with typical urticaria and angio-oedema manifestations.13 The hives can persist for more than six months and be located in different parts of the body1,2. They may vary in size and are typically intensely itchy.12 Angio-oedema is frequently found in patients with CAU, it is characterized by pronounced swelling of the dermis and sub cutis and it usually affects mucous membranes.1,2,12
Patients with positive autologous serum skin tests have more severe attacks, the severity is based on the frequency, duration and hive extension in individual episodes.32,33
During the follow up of patients with CAU a great number of diagnostic tests are usually performed and most of them are expensive and time consuming. In 58 % to 70 % of cases the cause is idiopathic34. This can generate stress and anxiety in the patient who has the need to know the etiology of the disease.
Confirmation of the presence of functionally autoantibodies brings relief in some cases to both the patient and the physician, and in severely affected individuals if conventional therapy has not been efficient then immunomodulatory therapy could be initiated.
DiagnosisIn our country we utilise autologous serum skin test as a screening tool in all patients with CIU to find CAU.35,36 This is an easy to do inexpensive test which, when performed correctly, has a great sensibility in detecting autoantibodies in children (78 %-85 %) especially in places where the histamine release by basophils test cannot be performed.
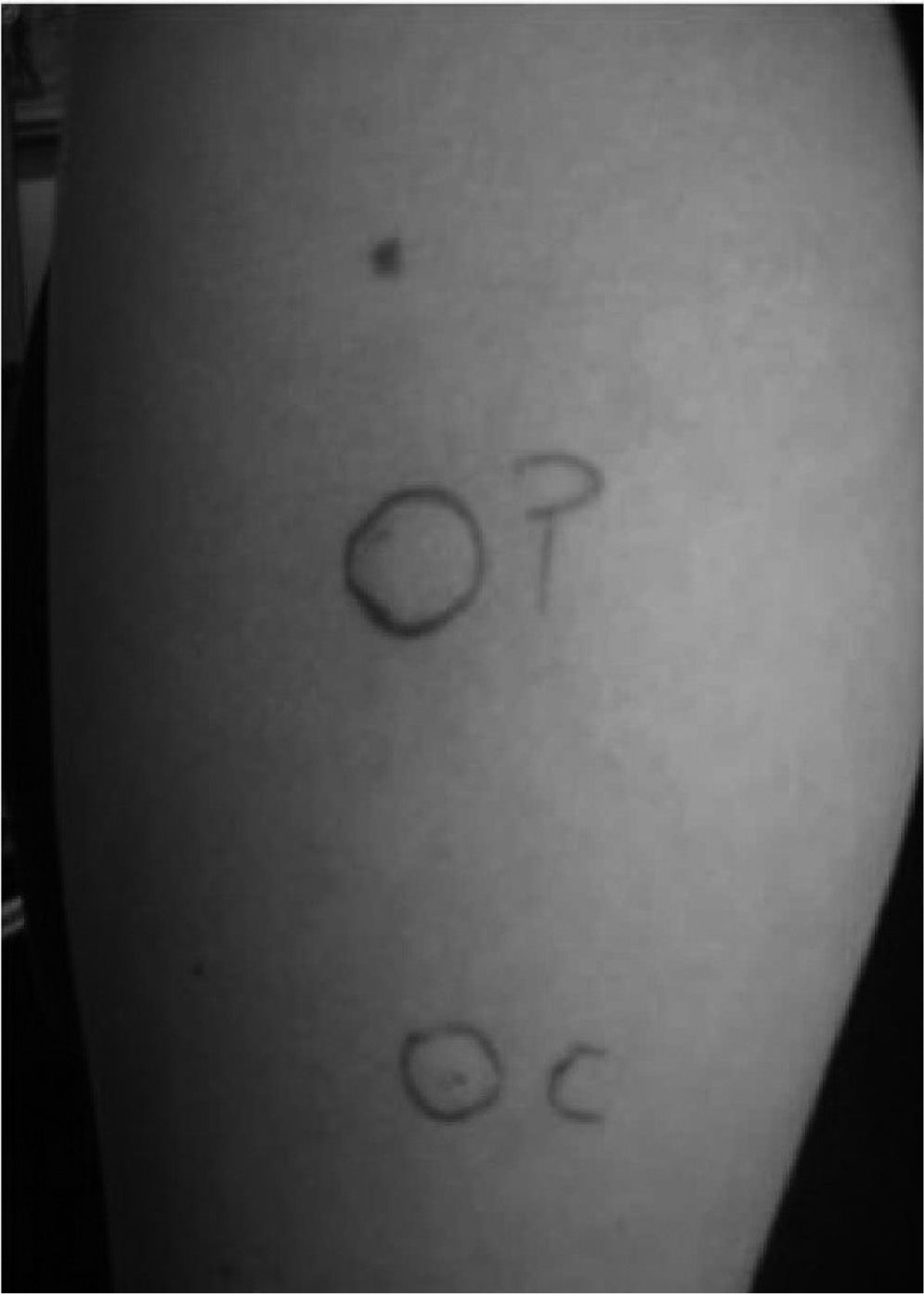
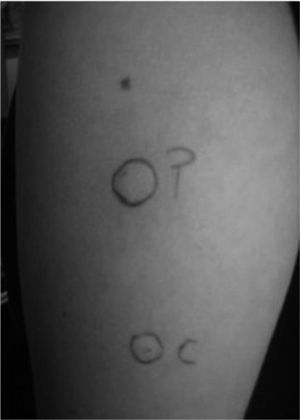
The test consists in the intradermic application of 0.05 of the patient's serum; as well as saline and histamine for control. A wheal 1.5mm greater than that obtained with the saline after 30 minutes is considered a positive reaction (fig. 3).
A correlation exists between the test and the duration and severity of the disease.37 In some studies there appears to be a relation with the presence of IgG antibodies against Helicobacter pylori38 although this relation is not found in other studies.39
In specialised centres the positive reaction is confirmed by the in vitro demonstration of the presence of histamine releasing substances from basophils and dermic mast cells. There is an ongoing search for a more specific and sensible screening test. Basopenia due to sequestration of basophils into affected skin, CD 63 expression and basophils activity markers detected by flow cytometry are promising screening tools for the future.40–44
TreatmentThe primary treatment of urticaria is the removal of the inciting agent (when identifiable) and symptomatic relief. Oral firstand second-generation antihistamine agents are the cornerstones of therapy. Levocetirizine appears to be safe in children as young as 12–24months of age.45 Fexofenadine and desloratidine are approved for use in children (they are safe and have a good tolerability).46,47
In our patient symptoms became worst at nights, which lead us to initiate hydroxyzine 25mg associated with loratadine 10mg in the morning. Life style changes such as the avoidance of NSAIDS, profuse sweating and thick clothes were instituted. Due to low response to treatment fexofenadine 240mg/day was initiated.
The addition of leukotriene inhibitors such as montelukast may be of some benefit,43,48 but studies examining the effectiveness of these agents for chronic urticaria have produced conflicting results.49 In our patient, the addition of montelukast 10mg/day produced no benefits.
Personal and economic factors played an important role in the development of exacerbations that consisted of dyspnoea and a sensation of thoracic oppression that required the use of prednisone 20mg/day for 5days with a gradual decrease in the dose over the following 5 to 6days.
CU can produce a decline in the quality of life8,17 similar to that seen in three-vessel coronary disease. Due to the partial response to treatment that our patient exhibited we considered the initiation of immunomodulatory therapy. In a double-blind placebo controlled study cyclosporine has proven to be effective in patients with autoantibodies.11 Doses of 2–5mg/kg/day relieve itching in a few days and stop the development of new skin lesions. Treatment lasts 3 to 4months. One third of the patients remain in remission and one-third relapse, but can be quickly controlled with the use of antihistamines at conventional doses. The final third of patients relapse and do not improve with the use of antihistamines; cyclosporine should be continued in these patients. Renal function, blood pressure, and lipid levels should be closely monitored when using cyclosporine.
Intravenous gamma globulin at a dose of 400mm/kg/day for 5days has been useful in small studies.10 In our experience gamma globulin has been effective in three adults, with CAU improving symptoms in two of them and a remission greater than six months after being suspended.
Recently the use of Anti IgE antibodies (Omalizumab) has been proven useful in the treatment of patients with CAU not responsive to antihistamines.50
CAU is not a common disorder in children and there is no consensus about the use of immunomoduladory therapy in children with CAU.
The presence of neurologic manifestations is extremely rare. The headache that our patient presented is an uncommon finding in patients with CAU and it could indicate a greater severity. The clinic neurologic examination was normal; however, no image studies were done in our patient.
Treatment represents a challenge since gamma globulin is an expensive treatment and it is not available in our country. We believe that studies searching for new effective and accessible treatments especially for third world countries should be initiated.