The term food allergy refers to the immune reaction (mediated by IgE or otherwise) that develops in response to the ingestion of a concrete type of food. Among the different potential manifestations of an allergic reaction, those exclusively affecting the gastrointestinal system are described.
In recent years, the study of non-IgE-mediated food allergy has grown in relevance. These disorders are almost always of a transient nature, inherent to (though not exclusive of) nursing infants, and with gastrointestinal symptoms that may have variable repercussions upon the nutritional state of the patient.
The prevalence of such reactions is not known, though some studies report that up to 60 % of all cases of allergy to cow's milk proteins (CMPs) are due to non-IgE-mediated mechanisms. The latency period between the time of ingestion and the appearance of the first clinical manifestations is greater than in the case of IgE-mediated reactions, and the underlying immunopathological mechanism has not been clearly established – although it is accepted that T cell mediation is involved.
The gastrointestinal problems derived from these delayed or chronic reactions comprise allergic proctocolitis, enterocolitis and food protein enteropathies.
These digestive disorders tend to appear in the first months of life, and are of a progressive and generally self-limiting nature, with resolution at about two years of age. The most commonly implicated food is milk and, in our setting, there have also been reports implicating fish, egg and rice – although such reactions can be triggered by any protein introduced into the infant diet.
These manifestations disappear after removing the causal protein from the diet. When the causal proteins are CMPs, a highly hydrolysed infant formula is supplied as substitute, and if the latter is not tolerated, an elemental amino acid-based formula is prescribed.
In the last few decades, the different allergic disorders have grown almost exponentially in the industrialised world. The reasons for this increase are not fully known, although a number of hypotheses have been proposed. One such hypothesis postulates that excessive measures of hygiene serve as a triggering factor causing the insufficiently stimulated host immune system to defend the body from agents which a priori are harmless.
In the case of food allergy, the latest studies show that in the United States, up to 4 % of all adults and 8 % of all children under three years of age suffer food allergy1,2 — this prevalence is much greater than that recorded by Sampson five years earlier.3,4 On the other hand, the prevalence of allergy to certain concrete foods is growing at an alarming rate; this is the case of peanut allergy, which has doubled in frequency in the last five years in North America.5 The Alergologica 2005 study collects the data related to the Spanish population, and concludes that in this country that almost one out of every 10 children under four years of age, and 2 % of all adults, suffer some allergic reaction to food proteins.6
Recently, the European Academy of Allergy and Clinical Immunology (EAACI) published a position document, revising the nomenclature of allergic processes. A series of terms were proposed for use, independently of the target organ involved and of the age of the patients experiencing allergic disease. The document in question is based on current knowledge of the mechanisms that trigger and mediate in allergic reactions7 (Table I).
The term food allergy refers to the immune reaction (mediated by IgE or otherwise) that develops in response to the ingestion of a concrete type of food. In this sense, food allergy represents an abnormal response of the immune system of the gastrointestinal mucosa to antigens arriving via the oral route.
The manifestations of food allergy can range from very mild situations to severe shock. In addition, different organs or systems can be affected, such as the oral cavity; gastrointestinal tract; skin; respiratory system; and cardiovascular system. Of the different manifestations that can be triggered as a result of an allergic reaction, description is made of those exclusively affecting the gastrointestinal system.8 Regardless of the immune mechanism involved, the different presentations of gastrointestinal hypersensitivity are clinically quite similar, as they share many symptoms, although they tend to differ in terms of the timing of onset, severity, and duration.9
From the pathogenic perspective, in most allergic reactions of the digestive tract the immunological nature of the manifestations is not demonstrated, only assumed. The only clearly identified mechanism refers to immediate hypersensitivity reactions mediated by IgE antibodies, with the activation of mast cells (also known as type I reactions). Delayed or late non-IgE-mediated type IV immune reactions in turn are the result of cellular mechanisms that prove difficult to demonstrate. There are also other immune reactions to foods that can implicate several IgE-mediated and non-IgE-mediated pathogenic mechanisms.10,11
From the pathogenic perspective, food allergy reactions can be classified into three groups:9,10
IgE-mediated reactionsThe symptoms in this case develop minutes or even seconds after ingestion of the causal food, and practically always within the first two hours after ingestion. The different reported manifestations range from immediate gastrointestinal hypersensitivity to oral allergy syndrome or anaphylaxis. The underlying pathogenic mechanism has been well established and involves type I reactions with the intervention of IgE antibodies specifically targeted to a concrete food. These reactions are easily demonstrated by immediately readable skin tests, with serum quantitation of the antibody titres.
Mixed IgE-mediated / IgE-non-mediated reactionsThese reactions comprise the primary eosinophilic gastrointestinal disorders: oesophagitis, gastroenteritis (gastritis/ enteritis) and eosinophilic colitis. Although the precise etiopathogenic mechanism is not clear, there is evidence to suggest an allergic basis for these disorders. Their prevalence in the industrialised world has experienced an increase comparable to the growth of allergic problems in the western population, and approximately 81 % of the affected patients are atopic. The diagnosis is based on the presence of three criteria: gastrointestinal symptoms, an eosinophilic infiltrate in one or more gastrointestinal regions, and the absence of any other potential cause of tissue eosinophilia. Peripheral eosinophilia is found in over 50 % of these cases.
Non-IgE-mediated reactionsIn recent years, the investigation of non-IgE-mediated food allergy has grown in relevance, and has been the subject of many works and publications. These disorders are almost always of a transient nature, inherent to (though not exclusive of) nursing infants, and with gastrointestinal symptoms that may have variable repercussions upon the nutritional state of the patient.
The current prevalence of such reactions in the paediatric population is difficult to establish, although some studies report that up to 60 % of all cases of allergy to cow's milk proteins (CMPs) are due to non-IgE-mediated mechanisms.1
The latency period between the time of ingestion and the appearance of the first clinical manifestations is greater than in the case of IgE-mediated reactions, and the underlying immunopathological mechanism has not been clearly established — although T cell mediation is accepted to be involved. The gastroenteropathies derived from these delayed or chronic reactions comprise allergic proctocolitis, enterocolitis and food protein enteropathies.
These digestive disorders tend to appear in the first months of life, and are of a progressive and generally self-limiting nature, with resolution at about two years of age. The most commonly implicated food is milk and, in our setting, there have also been reports implicating fish, egg and rice — though such reactions can be triggered by any protein introduced in the infant diet.
Proctocolitis is characterized by the presence of red blood mixed with the stools in healthy breastfed infants or infants receiving artificial formulas, and subsides after withdrawal of the milk proteins, or upon introducing special formulas (with hydrolysed proteins, or based on amino acids). The underlying mechanism is not known, though IgE is clearly not implicated. Endoscopy is known to show focal or diffuse colitis, with oedema and erosions. The biopsy reveals eosinophil infiltration and occasionally lymphoid nodular hyperplasia.
Enterocolitis is characterized by incoercible vomiting that can be accompanied by diarrhoea, leading to dehydration and hypotension, and even lethargy and shock. These manifestations improve with intravenous fluid therapy, and the condition disappears when the causal protein has been eliminated from the diet. When CMPs are the cause, a highly hydrolysed infant formula is supplied as substitute, and if the latter is not tolerated, an elemental amino acid-based formula is prescribed. The underlying immune mechanism is mediated by T cells.
In an in vitro study, Van Sickle et al.12 observed that in children with protein-induced enterocolitis syndrome, peripheral blood mononuclear cell stimulation by the causal antigen induced greater cell proliferation than in children with negative test findings. Examined retrospectively, these data point to the presence of an immunologically mediated response (allergy) rather than intolerance (food hypersensitivity without an immune basis). Hoffman13 also reported a lymphocyte proliferative response in affected children, though without significant differences versus the control group. In any case, the physiopathological ramifications and clinical implications of these findings remain unclear. Some authors such as Heyman14 postulate a T cell-mediated response, in which proinflammatory cytokine (TNF-α) release would alter tissue permeability. In this way, increased passage of the antigen into the submucosa would be favoured, followed by the activation of antigen-specific lymphocytes. In patients with cow's milk-induced gastrointestinal reactions, increased concentrations of TNF-α have been found in stools following a positive milk test.15 Chung et al.16 recorded an increased presence of TNF-α in children with villous atrophy compared with those showing no such atrophy, and with the control group. It is known that the regulatory cytokine TGF-β1 protects the epithelial barrier of the gastrointestinal tract against the penetration of foreign antigens. A reduction also has been reported in the type 1 (but not type 2) TGF-β1 receptor population in duodenal biopsies of patients with enterocolitis. In any case, further studies are needed to establish the immune basis of these diseases, and in this context a deficient TGF-β1 response and an excessive TNF-α response may be important factors to be taken into account.
The food protein enteropathies are characterized by chronic diarrhoea, vomiting and arrested body weight gain. In the same way as in the above-mentioned disorders, cellular immune mechanisms intervene — in this case causing patchy and nonspecific inflammation and damage to the walls of the digestive tract. Untreated children develop tissue changes, with moderate or intense partial atrophy and moderate intraepithelial lymphocyte infiltration (without eosinophils). An increase is seen in the activated CD4+ cell count within the lamina propria of the mucosa of the small bowel, as well as an increased presence of intraepithelial CD8+ cells.3 These tissue changes lead to protein-losing enteropathy, with secondary hypoalbuminemia, complement depletion, oedemas, anaemia and other signs of denutrition. The incidence of such disorders appears to have decreased in recent years. The most common causal food is cow's milk, although there have been reports involving soy, egg, fish and cereals. Cow's milk enteropathy usually resolves at around two years of age, although in some cases the problem may persist throughout childhood.11,17
Celiac disease (CD) would be the variant triggered by gluten proteins (gliadins, secalins, hordeins, and possibly avenins), found in genetically susceptible individuals and characterized by severe atrophy of the proximal small bowel mucosa. Such intolerance is permanent, and the intestinal mucosal damage results in impaired absorption and nutrient utilisation (basic elements, salts and vitamins). The clinical and functional repercussions in turn depend on the age and physiopathological conditions of the patient. It seems that factors such as the absence of breastfeeding, the ingestion of large doses of gluten, and the early introduction of cereals in the diet of susceptible infants could be risk factors for CD. The clinical characteristics of CD vary according to patient age at presentation. In this sense, intestinal manifestations and slowed body weight and height gain are more common when the diagnosis is established in the first years of life. However, when the disease develops later in childhood, extraintestinal symptoms appear in the form of low height, delayed puberty, ferropenic anaemia, osteopenia, or autoimmune diseases associated to CD (thyroiditis, type 1 diabetes mellitus, etc.).
Most models of the pathogenesis of CD consider the latter to be an immune disorder in which a combination of genetic and environmental factors must intervene in order for the disease to develop. A strong association has been found between the genes that encode for the class II HLA molecules and CD. Specifically, the heterodimer molecule DQ2 (alleles DQA1*0501 B1*0201) has been shown to be present in 95 % of all affected patients.
Celiac disease may be clinically silent or even latent, with an initially normal intestinal mucosa despite the consumption of gluten, in certain genetically susceptible individuals. Malignization is the most serious potential complication, and is determined by the sustained presence of gluten in the diet, even in small amounts. Thus, a strict gluten-free diet is the key to the management of CD, and must be recommended for the full life of the patients, regardless of whether they have symptoms or not. A strict gluten-free diet leads to the disappearance of the clinical manifestations and functional alterations, with normalisation of the intestinal mucosa.10
Gastrointestinal manifestations of non-IgE-mediated food allergy: proctocolitis and enterocolitisAllergic proctocolitisAllergic proctocolitis was first described by Rubin18 in 1940, and subsequently by Gryboski19,20 in 1966 and 1967. This disorder is characterized by inflammatory alterations of the colon and rectum, secondary to an immune reaction triggered by the ingestion of foreign proteins. The prevalence and natural history of allergic proctocolitis is not clear, though its frequency appears to be increasing in our setting, even in infants who are exclusively breastfed.21
The clinical picture develops in the first weeks or months of life (neonates and infants between 2days and 3months old, and practically always within the first 6months of life). The symptoms are always gastrointestinal and comprise rectal bleeding, in most cases associated to diarrhoea with mucus — although the stools may also appear normal. Haemorrhage can range from small spots of blood mixed with the stools to abundant bleeding (rectorrhagia). Bloody stools can increase gradually, with the erratic appearance of blood for several days, followed by the presence of blood in most bowel movements, until the causal agent is withdrawn. The general condition of the child is not affected, there is no arrest or loss of body weight, and abdominal palpation reveals no alterations.10,21,22
The laboratory test results are normal in most infants, though it is possible to detect discrete alterations in the form of anaemia, hypoalbuminaemia or peripheral eosinophilia in isolated cases.
A number of foods have been associated to allergic colitis (soy, fish, egg, wheat, etc.), although cow's milk is implicated in almost all cases. Approximately 60 % of all cases of proctocolitis are found in breastfed children.23–26 The triggering allergens in these cases are CMPs excreted in breast milk after the ingestion of dairy products by the mother. As a rule, the most allergenic protein is p-lactoglobulin.27 The rest of the affected patients correspond to infants fed formulas containing CMPs or soy. Odze28 reported that up to 30 % of all patients are allergic to both proteins (i.e., CMPs and soy).
The risk factors for the development of allergic colitis are an immature immune system, altered intestinal permeability and other factors that activate focal immune function, such as genetic susceptibility in combination with particularly sensitising foods (milk, egg, fish, nuts, soy).26
Unfortunately, there are no non-invasive, specific diagnostic tests, and the existing laboratory or biochemical techniques lack the required specificity and sensitivity. Ultrasound is able to evidence thickening of the mucosa. The skin tests and specific IgE titres are negative. The diagnosis is fundamentally based on a detailed case history and patient response to the elimination of suspect proteins from the diet (generally cow's milk), after ruling out other possible explanations for the clinical manifestations such as infection, necrotizing enterocolitis, or anal fissures or invagination. Most cases are diagnosed and treated on an empirical basis. In these children, re-exposure (provocation test) should be contemplated in order to confirm the diagnosis. Xantacos29 confirmed allergic proctocolitis via endoscopy and biopsy in only 64 % of healthy infants consulting for rectal bleeding. Rectosigmoidoscopy and biopsy are not required on a routine basis, provided that the patient responds well to withdrawal of the protein in question. If this is not the case, however, then at least endoscopy must be carried out. The endoscopic findings comprise an oedematous and erythematous mucosa with possible superficial erosions or ulcerations, bleeding and lymphoid nodular hyperplasia. The affected surfaces (particularly descending zones and sigmoid colon) alternate with healthy areas (patched lesions). The histological study of the rectal biopsy reveals an eosinophilic infiltrate in the full thickness of the mucosa and lamina propria (over 20 cells per high-magnification field) and, less frequently, cryptic abscesses.24,26
Treatment consists of eliminating the suspect proteins from the diet (generally CMPs). In the case of breastfed infants, if the desire is to maintain breastfeeding, the suppression of dairy products from the maternal diet will gradually resolve the symptoms in most patients. In isolated cases a soy and egg exclusion diet is also needed. If the condition does not begin to subside within 48–72 hours, a hydrolysed protein infant formula should be considered, and if this does not improve the situation then an elemental amino acid-based formula is indicated. In infants fed artificial formulas, the introduction of a special formula is indicated. The recommendation in this case is a hydrolysed protein formula (it should be remembered that 30 % of all patients with reactions to CMPs also react to soy proteins), and if this proves ineffective, then an elemental formula (amino acids) is advised.
These treatment measures are only temporary, since it must be taken into account that proctocolitis is a benign and self-limiting disease in which the infants by one year of age are able to tolerate a free diet, and the long-term prognosis is excellent30 (Table II).
Principal characteristics of allergic proctocolitis
|
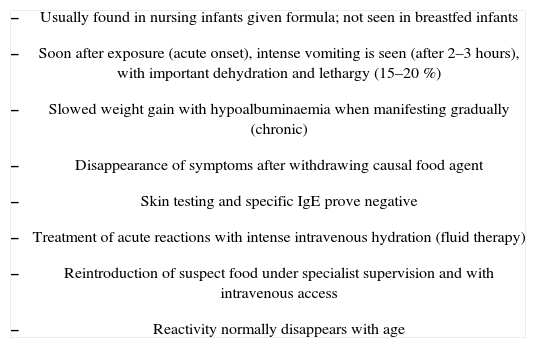
Enterocolitis syndrome induced by food proteins usually develops in the first six months of life. The condition generally manifests with diarrhoea as the most common problem, ranging from soft and abundant stools to liquid and explosive diarrhoea. Some patients may experience very intense vomiting and diarrhoea 2–3 hours after ingestion, and in the most severe cases the situation leads to dehydration, arterial hypotension, lethargy (15–20 %) or even shock.31 Colon participation would give rise to blood in stools. The infants can also present irritability and abdominal bloating of a nonspecific nature, or a flattened ponderal curve. There may be signs of malabsorption and arrested growth.10,23
The symptoms seen in nursing infants with protein-induced enterocolitis are similar to, but more severe than, those of protein-induced enteropathy. Since both bowels (large and small) are usually affected, the term “enterocolitis” is used. Other possible causes of non-allergic enterocolitis (infectious, neonatal enterocolitis) must be ruled out in all cases.21
The most frequently implicated food is cow's milk. In these cases, the symptoms appear in the first months of life, and generally in a gradual manner. Breastfeeding appears to be a protective factor against food protein enterocolitis. To date there have been no reports of the condition manifesting in infants who are exclusively breastfed; indeed, the acute disorder manifests when cow's milk is introduced in the infant diet. However, when artificial formulas are used from the first days of life, the symptoms appear in a more insidious manner — affecting the progression of body weight and height, and inducing hypoalbuminaemia, together with the characteristic vomiting and diarrhoea.
In addition to cow's milk, cases of enterocolitis have been associated to soy proteins and solid foods such as egg, legumes, oats, rice, barley, potato, squash, fish, chicken and turkey. In some cases the patients concomitantly exhibit reactivity to more than one food.32–34
The diagnosis of food protein enterocolitis is based on the case history and on the patient response to withdrawal of the causal allergen. The endoscopy findings and intestinal biopsy results obtained in some patient series have reported only nonspecific alterations. Cryptic abscesses appear in the colon, along with a diffuse inflammatory infiltrate with the presence of plasma cells. The small bowel in turn shows wall oedema, acute inflammation, and mild villous atrophy. Some cases may present focal erosive gastritis and oesophagitis, with important eosinophilia and more accentuated villous atrophy.35,36
The blood tests may exhibit eosinophilia without other characteristic alterations. This finding and the concomitant worsened patient general condition and lethargy may lead us to suspect the start of sepsis. The more severe cases exhibit hyponatremia, acidosis and even metahaemoglobinaemia. The latter is related to increased haem group oxidation due to an increase in intestinal nitrite levels secondary to lesser catalase activity during the inflammatory process.37 The stools may contain blood or prove reducer-positive. The skin tests and specific IgE antibodies are typically negative,34 and in those cases which prove positive (a small proportion), the probability of developing tolerance may be lower.38
Diagnostic confirmation is based on resolution of the clinical picture after withdrawing the causal food, and on reappearance of the manifestations on repeating oral exposure. Such testing must always be performed under medical supervision in the hospital setting, and in severe cases, an intravenous catheter must be placed first.
Recommended treatment consists of hydrolysed proteins, which offer a favourable course in practically all cases. It should be remembered that 50 % of the children who react to CMPs will also react to soy proteins — as a result of which hydrolysed proteins should be indicated directly as first choice. In those infants which fail to respond favourably to such measures, and also react to hydrolysed proteins, an elemental amino acid-based formula should be provided.39
Treatment of the acute process involves the administration of intravenous fluids. Corticosteroids may help arrest the cellular immune response that appears to be implicated in the process, and are considered useful in patients with a history of very severe reactions. Exceptionally, adrenalin or antihistamine dosing may be necessary due to the possibility of concomitant IgE-mediated allergy or serious cardiovascular reactions.
An important consideration is patient and family education regarding the preventive measures needed to avoid the causal foods, and as refers to the actions to be taken in the event of accidental ingestion.
If the disease is produced by CMPs, resolution tends to occur at about one year of age, while in the case of other causal foods such as fish, chicken or rice, the disorder may persist throughout infancy.
The children typically acquire tolerance of the causal food with increasing age (mostly towards 2–3years of age), though a small proportion of subjects may maintain hypersensitivity throughout infancy.40 Reintroduction of the causal food must be done in the hospital setting, with the preparation of a venous access and fluid therapy, in order to ensure prompt treatment in the event of clinical reactivity (Table III).
Principal characteristics of food-induced enterocolitis syndrome
|
Food protein-induced enteropathy tends to develop in the first two years of life, although most affected children develop the symptoms before 12months of age.11
Clinically, the condition manifests as diarrhoea and vomiting in the nursing infant, a few weeks after introducing the causal food. The patients often develop malabsorption syndrome, which affects the progression of body weight and height (the former generally being more affected than the growth in height).17 In some cases associated protein-losing enteropathy is observed, with hypoalbuminaemia, oedemas and increased serum α1-antitrypsin levels.41
The most common causal food is cow's milk, though there have been cases attributed to soy, rice, chicken, egg and fish.32,33,42
The clinical manifestations and pathogenesis of food protein-induced enteropathy are very similar to those of celiac disease. However, unlike the latter, food protein enteropathies resolve at about two years of age and do not entail an increased risk of malignization of the intestinal lesions.43
The histopathological and immune studies indicate cellular immune-mediated damage of the intestinal mucosa, though the mechanism is not well known. The laboratory tests often evidence malabsorption with moderate anaemia, ferropenia, hypoproteinaemia or vitamin K deficiency. Most patients suffer moderate steatorrhoea and lactose intolerance, which improve upon withdrawing the causal food.17
The intestinal biopsy in turn shows patchy lesions with variable grades of villous atrophy, crypt hyperplasia, an increased presence of intraepithelial lymphocytes and, in some cases, a mild eosinophil infiltrate.44
The immunohistochemical study in turn shows an increase in activated CD4+ cell count in the lamina propria of the small bowel, along with intraepithelial CD8+ cells that normalise with the exclusion diet and return to levels similar to those observed before provocation.17
In the same way as in proctocolitis and enterocolitis caused by food proteins, no increased titres of specific IgE against the causal food are seen. Thus, the skin tests and serum IgE determinations are nonspecific in the absence of an associated IgE-mediated food allergy disorder.45
The diagnosis is based on the combination of a detailed case history and the provocation tests. An intestinal biopsy may provide information of use in application to the diagnosis and follow-up.
Treatment consists of withdrawing the causal food from the patient diet. When CMPs are responsible for the problem, extensively hydrolysed or soy formulas should be provided — though it must be remembered that up to 15 % of all affected children can develop symptoms in response to this food.