Editor: D. Solé
IntroductionAllergic diseases are diagnosed and treated according to clinical history and physical exam. Complimentary assessment to verify allergic sensitisation or immediate-type hypersensitivity should be done in vivo or in vitro by the determination of specific IgE antibodies.
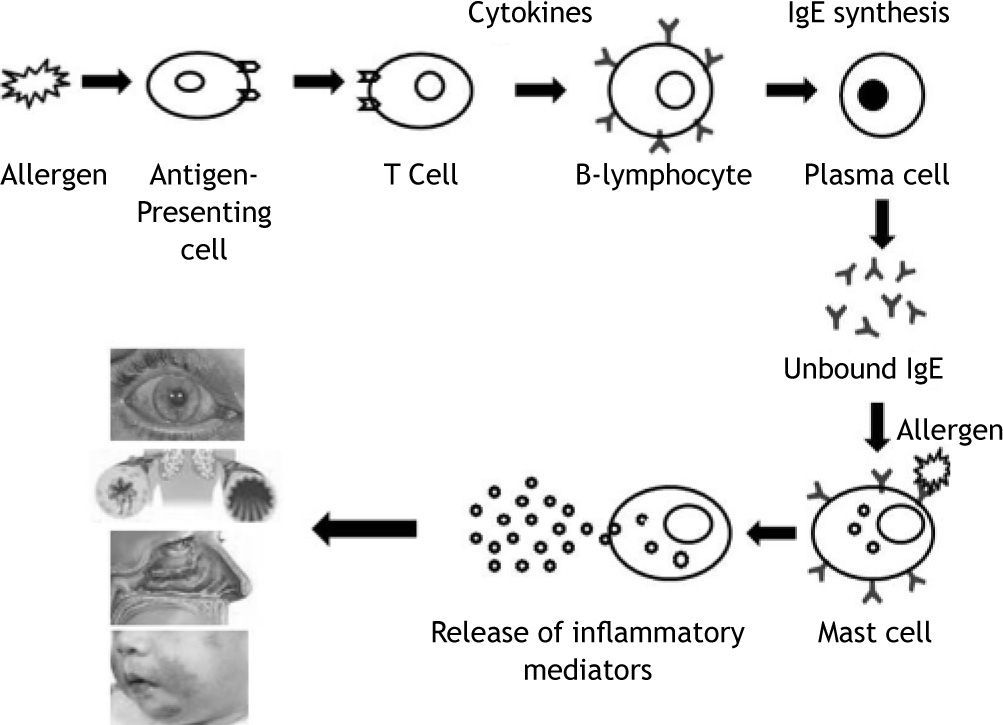
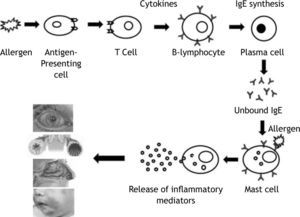
Immunoglobulin E is produced by the interaction of a number of cell types following the introduction of antigens through inhalation route, skin or parenteral exposure. Once taken up by antigen-presenting cells, antigen is processed and presented to T helper cells. These T cells subsequently secrete a number of cytokines that cause B-cell lymphocytes to proliferate and in some cases produce allergen-specific IgE antibody. These specific IgE binds onto Fc∈ receptors on a number of cells, particularly tissue mast cells and circulating basophils, creating a state of sensitisation. Subsequent allergen exposure causes mast cell surface bound IgE antibody to be cross-linked, leading to an increase in intracellular calcium and the release of both preformed mediators (eg. histamine, proteases) and newly synthesized lipid derived mediators (eg. leukotrienes and prostaglandins). These mediators induce physiological and anatomical changes that cause allergic symptoms1. Measurement of specific IgE antibody for diagnosis and treatment of allergic diseases prompted this review.
In vivo specific IgE measurementSkin testing is a primary confirmatory test for allergen-specific IgE antibody that is used in diagnosis of human allergic disease. The allergen extract is introduced into the skin either by prick/puncture or intradermal injection. These tests have higher sensitivity, but lower specificity than in vitro tests and should be performed in all patients without restrictions of age.
Prick-puncture testsLewis and Grant described prick testing for the first time in 1924 and it was modified by Pepys in 1970. This is the easiest, fastest, and less expensive method to identify specific IgE antibodies. Drops of different glycerinated allergen extracts, placed on the volar surface of the forearm are passed by hypodermic needle at a low angle with the bevel facing up into the epidermal surface. The needle tip is then gently lifted upward to elevate a small portion of the epidermis not inducing bleeding. One minute later the solution may be wiped away with paper tissue or cotton. To avoid mixing of solutions a separate needle must be used for each test. Needles can be substituted for other devices, standardised needle, as single-point or multi-test. The immediate reaction (wheal and erythema) must be read at 15 to 20 minutes. A positive and negative control, histamine 10mg/ml and saline, respectively, need to be used. A positive test is considered when diameter wheal is greater than 3mm.
Several factors contribute to variability in the prick test results. Drops too close together (< 2cm apart from each other) cause overlapping reactions. Bleeding and dermographism can lead to a false-positive wheal and erythema. False-negative tests are consequences of antihistamine use, low potency of extracts and poor technique1,2.
Intradermal testsMantoux described the intradermal test and it is still used in clinical practice. Allergen can be administered intracutaneously through a 26- to 27-gauge needle. A volume of 0.01 to 0.05ml is injected to produce a small superficial bleb with 2 to 3mm in diameter. Before injection, air bubbles should be eliminated to avoid splash reactions. The syringe is placed at a 20° angle to the skin, and the bevel of the needle is angled downward, facing the skin, and penetrating entirely and superficially in the skin. The intradermal test requires 1000-fold lower concentration of antigen than the puncture test does to produce skin reactions about the same size.
Larger volume injected, tests to close to each other, high concentration and spreading erythema can lead to false-positive results. Subcutaneous injection results in false-negative test1,2.
In vitro specific IgE measurementSerum allergen-specific IgE antibody determinationAfter the discovery of IgE, advances in technology have provided new laboratory tools for the quantification of allergen-specific IgE antibodies in serum and on surface of basophils/mast cells. In vitro tests offer numerous advantages such as precise quantification, a lack of drug interference, safety, and long-term storage of specimens.
Quantitative immunoassays for IgE antibodies may be an adjunct to skin tests. In cases of food allergy among children with atopic dermatitis, cut-off values for IgE antibody concentrations to egg, milk, peanut and fish have been derived to provide 95 % positive and 90 % negative predictive values. There is correlation between results of inhalation challenge and specific IgE antibody to cat in sensitised subjects to this allergen.
Allergen-specific IgE antibodies are measured in the presence of other antibodies of the same isotype and antibodies of different isotypes yet specific for the same allergen. This requires specific recognition by the allergen-binding sites (Fab) and the isotype-specific epitopes (Fc) in the same assay. All assay designs therefore include a solid phase for the separation of bound and unbound IgE antibodies. The allergen source materials used in the assay should be well characterized, and critical allergens should not be lost during the production of reagents; this provides precise and reproducible data in clinical allergy investigation.
The solid-phase (allergosorbent) or liquid-phase allergen reagent is the principal component of the assay that confers specificity on the IgE antibody assay. It is the most complex and highly variable reagent in IgE antibody assays, in part due to the heterogeneity of most allergen extracts and the different chemistries used to insolubilise or label the allergenic proteins. In an attempt to improve on the antibody-binding capacity of the paper disc, a variety of carbohydrate-based allergosorbents (other than sephadex and paper), such as microcrystalline cellulose and agarose, were historically used in research. The most significant advance on clinical assays, however, was the development of an encapsulated hydrophilic carrier polymer to which allergen was covalently coupled. This polymer was configured into the shape of a small cup and called a CAP. Its allergenic protein-binding capacity was both superior to the paper disc and more user friendly than agarose or cellulose particles. Its use in the Pharmacia CAP system improved the allergosorbent's overall antibody-binding capacity, which led to more rapid assay kinetics and enhanced assay sensitivity.
Comparison among in vivo and in vitro tests for specific IgE
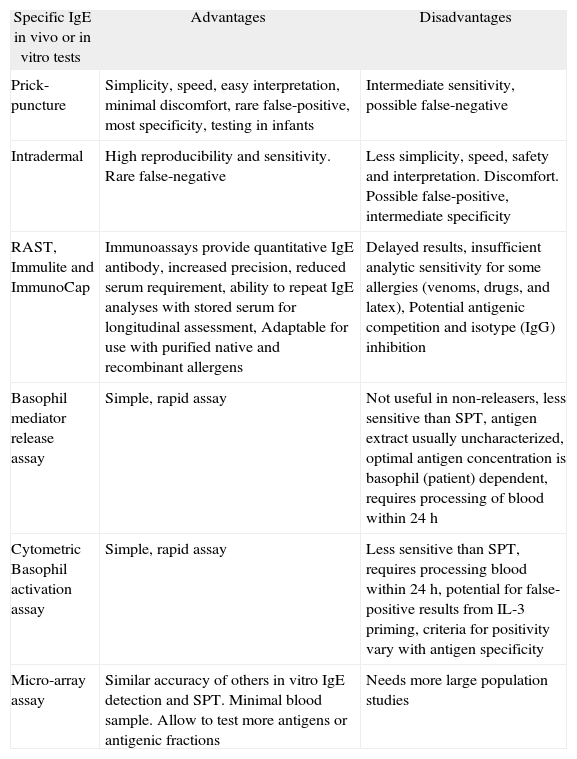
| Specific IgE in vivo or in vitro tests | Advantages | Disadvantages |
| Prick-puncture | Simplicity, speed, easy interpretation, minimal discomfort, rare false-positive, most specificity, testing in infants | Intermediate sensitivity, possible false-negative |
| Intradermal | High reproducibility and sensitivity. Rare false-negative | Less simplicity, speed, safety and interpretation. Discomfort. Possible false-positive, intermediate specificity |
| RAST, Immulite and ImmunoCap | Immunoassays provide quantitative IgE antibody, increased precision, reduced serum requirement, ability to repeat IgE analyses with stored serum for longitudinal assessment, Adaptable for use with purified native and recombinant allergens | Delayed results, insufficient analytic sensitivity for some allergies (venoms, drugs, and latex), Potential antigenic competition and isotype (IgG) inhibition |
| Basophil mediator release assay | Simple, rapid assay | Not useful in non-releasers, less sensitive than SPT, antigen extract usually uncharacterized, optimal antigen concentration is basophil (patient) dependent, requires processing of blood within 24h |
| Cytometric Basophil activation assay | Simple, rapid assay | Less sensitive than SPT, requires processing blood within 24h, potential for false-positive results from IL-3 priming, criteria for positivity vary with antigen specificity |
| Micro-array assay | Similar accuracy of others in vitro IgE detection and SPT. Minimal blood sample. Allow to test more antigens or antigenic fractions | Needs more large population studies |
Currently, there are five assays used clinically to detect allergen-specific IgE in human serum. On the basis of data from the College of American Pathologist's Diagnostic Allergy Proficiency Survey, three of these, the chemiluminescent assay from Hitachi Chemical Diagnostics (formally MAST), the Hycor Hy-Tech EIA (formally the Ventrex RAST), and the Thabest IgE, are infrequently used first-generation technologies that report qualitative (positive–negative) or semiquantitative (class-grade) results. The Pharmacia CAP system and the Diagnostic Products Corporation Alastat are second-generation assays that have achieved a high degree of quantitation, semiautomation, and good analytic performance. In recent years, both of these assays have transitioned to stand-alone, push-button, automated thirdgeneration systems known as the Pharmacia UniCAP and Diagnostic Products Corporation Immulite systems, respectively. The quality of allergen-specific IgE antibody measurements reported from clinical diagnostic allergy laboratories is not uniformly equivalent3,4.
To examine prospectively the extent of comparability among specific IgE results from different laboratories, Szeinbach et al. evaluated six diagnostic laboratories employing five different methods to assay specific IgE. Aliquots from 26 serum samples that contained variable levels of IgE specific to 17 common aeroallergens were sent in triplicate to each study laboratory during a 6-week time period. In all, 7,813 tests were analysed. Concordance among different assays in commercial use with one exception was not good. This was particularly true around the cut-off region where most assays demonstrated high inaccuracy. The Pharmacia CAP System used by two different laboratories demonstrated highly comparable results with good precision. Some assays were reproducible but not accurate. Others were neither reproducible nor accurate. The results of this study indicated that not all commercial laboratories/assays for specific IgE provide reproducible and accurate data. Significant potential for misdiagnosis was detected for some reported results5.
Wood et al. investigate whether similar results were obtained from Clinical Laboratory Improvement Act-certified laboratories that used three common systems for sIgE antibody determination with serum samples and mouse-human IgE chimeric antibodies with known specificity and quantity. Sixty samples for peanut and 20 samples for soy were submitted for sIgE determination on three different systems: ImmunoCAP, Immulite, and Turbo radioallergosorbent test (RAST). Mouse-human chimeric IgE antibodies specific for the major birch allergen Bet v 1 and for the dust mite allergen Der p 2 were also included. A qualitative evaluation using a cut-off of 0.35 kUA/L showed some differences in the ability to detect sIgE sensitisation, with the Turbo RAST being most variable. However, considerable differences were found with quantitative evaluation, with Immulite overestimating and Turbo RAST underestimating sIgE compared with ImmunoCAP. Similar discrepancies were seen with the mouse-human chimeric IgE antibody samples. These findings have potentially serious clinical implications, since each of these systems is widely used. It is therefore important that all laboratories inform which system they are using. Just because two systems present their results in the same units does not mean that the results are necessarily correct or interchangeable6.
Wang et al., to determine whether IgE levels derived from different assays are equivalent to those measured by ImmunoCAP, prospectively enrolled fifty patients from the Mount Sinai Pediatric Allergy practice. For each blinded sample, specific IgE levels were measured to egg, milk, peanut, cat, birch, and Dermatophagoides farinae in different laboratories, each using a different assay system (Phadia ImmunoCAP, Agilent Turbo-MP, and Siemens Immulite 2000). Results were analysed to determine whether IgE measurements were equivalent. Food allergen-specific IgE levels were correlated with clinical data and thresholds determined that predict probability of clinical disease in 50 % or 95 % of subjects. Variable degrees of agreement existed among the three assays. Immulite 2000 overestimated all specific IgE levels compared with ImmunoCAP. Turbo-MP overestimated for egg but underestimated for birch and D. farinae. Differences for milk, peanut, and cat were observed, without a trend toward overestimation or underestimation. Furthermore, several values for the food allergens were discrepant around the 50 % and 95 % positive predictive values for clinical reactivity. Discrepancies in specific IgE values from three different assays can potentially lead to altered management and treatment. The predictive values for clinical reactivity associated with food-specific IgE levels determined by ImmunoCAP should not be applied to results from other assays7.
In vitro chemical mediators releaseAllergen cross-linking of IgE on the surface of basophils induces the release of a number of mediators, including histamine and cysteinyl leukotriene C4. In addition to the need for rapid blood processing and the concern about non-releasers, these in vitro test results add little to the diagnostic predictive value offered by skin and provocation testing. Moreover, the performance of mediator release assays varies with the quality of allergen extracts available and the techniques (concentrations, incubation times, criteria for positivity, quality control reagents, and methods) used among laboratories3,4.
Upon challenge with a specific allergen, basophils not only secrete quantifiable bioactive mediators but also upregulate the expression of different markers which can be detected efficiently by flow cytometry using specific monoclonal antibodies. The technique has been applied in the investigation of IgE-mediated allergy caused by classical inhalant allergens, food, Hevea latex, hymenoptera venoms and drugs. It is also appreciated that the technique proves valuable in the diagnosis of non-IgE-mediated (anaphylactoid) reactions such drug hypersensitivity and the detection of autoantibodies in certain forms of chronic urticaria8.
A new era in the diagnosis of allergic diseasesStandardisation of allergenic extracts for diagnosis and immunotherapy is based on biological standardisation, i.e. IgE-binding potencies. Skin tests and competitive IgE-binding assays are important elements of allergen standardisation, especially from a safety perspective. However, biological standardisation does not provide specific information about the major allergen content of allergen vaccines, i.e. the content of the active ingredients needed for attaining efficacy of immunotherapy. Another disadvantage of the current system is that allergen manufacturers express potencies of their products in company-specific units that do not allow product comparison and this situation is not tenable as an international system of standardisation.
In the 1980s, the World Health Organization/International Union of Immunological Societies (WHO/IUIS) Allergen-standardisation Subcommittee developed International Reference Preparations (IRP) of several extracts to facilitate product comparison. However, these IRP were not adopted by the industry or by regulatory authorities. Most major inhalant allergens became available as recombinant molecules, and the dependence of effective immunotherapy on administration of defined quantities of major allergen had become well accepted. This system of allergen standardisation allows comparison of products and at the same time gives accurate information on the content of the active major allergens.
A European initiative (CREATE) is developing international standards of allergen extracts for diagnosis and treatment based on biological features, using sandwich enzyme-linked immunosorbent assays (ELISAs) for their accurate measurement. Purified recombinant allergens were used to compare with their natural counterparts serving as gold standards. rBet v 1, rPhl p 1, rPhl p 5a and rPhl p 5b, rOle e 1, rDer p 1, rDer p 2, rDer f 1 and rDer f 2 were compared with purified natural allergens for physicochemical (identity, purity, folding, aggregation state, solubility and stability) and immunological (IgE-binding potency, biological activity and dose–response behaviour in ELISA) characteristics.
Eighteen purified allergen preparations (eight natural allergens and ten recombinant versions) were produced by the CREATE consortium. This initiative seeks the best physicochemical characterization of allergens, like identity, purity, homogeneity, structure, aminoacid analysis, mass spectrometry and others.
Immune reactivity and biological activity were analysed by direct IgE-binding in RAST, direct binding in dot-blot, RAST-inhibition and biological activity in basophil histamine release with good concordance. Stability was satisfactory both in short as in long term9,10.
Application of a recombinant reference molecule in allergen-standardisation protocols is not sufficient to improve comparability of products on the basis of mass units of major allergen. Recombinant references need to be linked to one or two validated assays that have demonstrated natural and recombinant allergen with similar performance characteristics, thus leading to the same designation of potencies in mass units of major allergen11.
Since the identification and cloning of the first allergenic proteins in the late 1980s, thousands of allergens have been identified and their sequences determined. Nonetheless, Radauer et al. verified that allergens are distributed into few protein families and possess a restricted number of biochemical functions. They sought to build AllFam, a database of allergen families, and use it to obtain common structural and functional properties of allergens. Allergen data from the Allergome database and protein family definitions from the Pfam database were merged into AllFam, a database that is freely accessible on the Internet at http://www.meduniwien.ac.at/allergens/allfam/. The small number of protein families that contain allergens and the narrow functional distribution of most allergens confirm the existence of yet unknown factors that render proteins allergenic12.
An important task in allergy diagnosis and treatment should be the identification and characterization of allergens actually eliciting sensitisation and causing the disease. Thus far a significant number of molecules from different sources have been identified for inducing hypersensitivity reactions. The high structural similarity between phylogenetically conserved allergenic proteins present in different, apparently unrelated, sources seems to play an important role in IgE-mediated poly-sensitisation, which subsequently leads to immunological cross-reactivity and clinical syndromes. As many as 60 % of allergic patients are sensitised to pollen of more than one species. Extensive cross-reactivity within allergenic plants is likely to be caused by three families of widely distributed pan-allergens: profilins, calcium-binding proteins (CBPs), and nonspecific lipid transfer proteins13.
Cross-reactive carbohydrate determinants (CCDs) are probably the most widely occurring IgE epitopes. Approximately 20 % of allergic patients develop IgE antibodies against those glycans which appear incapable of provoking clinical symptoms despite their frequent presence in serum. Anti-CCD IgE does not cause allergic disorders because CCDs lead to induction of tolerance14,15. A novel carbohydrate epitope on a monoclonal antibody, cetuximab, has driven Th2 responses to food allergens.
The use of panels of recombinant allergens in diagnosis could help to delineate the reactivity profile of an allergic patient and determine whether one is co-sensitised to allergens from different sources or whether it cross-reacts with allergens that are present in these sources. Panallergens have been suggested as possible markers of multiple pollen sensitisations, and could be further used to predict cross-sensitisation/poly-sensitisation to several pollen allergens. Further studies using well-characterized recombinant allergens of the CBP and profilin family are necessary to understand more thoroughly and to predict sensitisation, IgE cross-reactivity and clinical relevance. Pan-allergens should be considered as diagnostic markers for poly-sensitisation and used in component resolved diagnosis13.
ConclusionDespite the major changes in immunology, prick test is still the gold standard for diagnosis of allergic diseases. There are important advances in the knowledge of molecular biology of allergens. In the near future, medical history, physical examination, prick tests and standardisation of allergens by molecular biology, will accurately improve diagnosis of allergic patients.
Financial disclosureNothing to declare