Allergen cross-reactivity between tobacco and other species of Solanaceae family (tomato, potato, aubergine and egg plant) have been reported. We have recently studied IgE response to tobacco in asthmatic patients sensitised to Lolium perenne (Perennial rye grass pollen) and have found that 30% of the tobacco responsive patients also have latex sensitisation.
ObjectiveThe aim of our study was to investigate the possibility of cross-reactivity between tobacco and latex in asthmatic patients with IgE response to latex.
MethodsA study was performed on tobacco and latex exposure in 15 patients who suffered from asthma and latex sensitisation and who were randomly chosen from our database of latex-sensitive patients. To identify tobacco and latex as possible allergens that might cause clinical specific responses, all these patients were tested with prick-tests, specific IgE to tobacco, latex and related allergens, bronchial challenge, and patch tests with tobacco, latex and nicotine. Immunological response was evaluated with immunoblotting, immunoblotting-inhibition and EAST-inhibition tests.
ResultsPositive prick and bronchial challenge with specific IgE>0.35kU/L to tobacco was demonstrated in 11 asthmatics who were also sensitised to rye grass. Tobacco IgE level was related with sensitisation to latex (p<0.002), but not to other vegetables belonging to the Solanaceae family. EAST-inhibition and immunoblotting-inhibition showed the existence of cross-reactivity between tobacco and latex.
ConclusionsCross-reactivity exists between latex and tobacco allergens. Smoker patients with IgE response to tobacco may be a risk population for latex sensitisation.
Several latex allergenic proteins have been purified and characterised, and some of them have been shown to be responsible for the so-called latex-fruit syndrome caused by the presence of cross-reacting homologous proteins in fruit and vegetables.1,2 Banana, avocado, chestnut and kiwi are the most frequently implicated foods in this syndrome, but association with several other fruits and vegetables, including pineapple, fig, passion fruit, mango, tomato, bell pepper, carrot, oregano, dill, aubergine and sage have been reported. Panallergens responsible for latex fruit syndrome are class I quitinases, which show cross-reactivity with latex due to N-terminal hevein domain. The allergen responsible for most cases of latex-fruit syndrome is hevein (Hev b 6.02), the amino-terminal fragment of prohevein.3 Plants produce defence-related proteins (DRPs) during stress response caused by microbial pathogens, chemical substances, mechanical damage, UV light and ozone.4,5 Many DPRs are cross-reactive allergens.6 Several DRPs resembling prohevein, a major allergen of natural rubber latex (NLR), can be bound by IgE from sera of patients allergic to NLR. Tobacco is widely used as a model in studies of stress responses.7–9 Allergenic cross-reactivity has been observed between tobacco and various other species of the Solanaceae family, which comprises many edible plants, such tomato, potato and eggplant.8 A 21-Kd protein of tobacco, purified by chitin affinity and reversed-phase chromatography, was bound by IgE from pooled sera of NRL-allergic patients.4,9
The relationship between environmental tobacco influence and tobacco hypersensitivity (measured by skin prick test, patch tests, specific IgE, immunoblotting and specific bronchial challenge tests) and clinical significance of a specific immunologic response to tobacco was investigated in 120 patients with obstructive pulmonary disease.10 In this study, positive tests with latex were found in 30% of asthmatics with clinical and immunological IgE responses to both tobacco and rye grass pollen.
Here, we investigate a possible cross-reactivity with clinical significance between tobacco and latex in asthmatic patients with IgE response to latex and tobacco.
MethodsPatientsWe studied the records of 1582 patients who suffered from asthma and had been admitted to our Allergy Department during the last year. We randomly selected 15 patients who suffered from sensitisation to latex. All patients were studied in the same way: a detailed clinical allergy history followed by skin tests with a complete battery of 32 allergens, including tobacco and latex extracts, specific IgE measured by CAP method and measurements of lung function. Sensitisation to tobacco and latex was regarded as the presence of: a) one or more positive skin tests (with a wheal of 3mm larger than the negative control to these allergens; b) a positive CAP test >0.35IU/ml and; c) a positive specific challenge. All these patients were informed of the objectives of the study and tested in order to try to identify tobacco and latex as risk-factors for bronchial inflammation. Possible sources of latex or tobacco contact were investigated with tobacco indexes and questionnaires, including job and surgical treatments in the past. Patch tests with tobacco, latex and nicotine were also performed. Immunological tests were carried out to test possible allergenic cross-reactivity among tobacco, latex and other related vegetable sources as members of Solanaceae family (tomato), fruits (Kiwi, melon, banana, avocado, pineapple, fig, passion fruit, mango) and vegetables (bell pepper, carrot, oregano, dill, aubergine).
Tobacco extractA commercial tobacco extract was supplied by BIAL- Aristegui Laboratoires, Bilbao, Spain (protein concentration 0.5mg/mL). We also performed extracts with fresh tobacco leaves, other fruits and vegetables related with latex, including Kiwi, melon, banana, pineapple, fig, passion fruit, mango, tomato, bell pepper, carrot, oregano, dill, aubergine and sage. Extract of latex was supplied from ALK-Abelló. Madrid, Spain, (protein concentration 0.5mg/mL), and purified hevein was kindly supplied from Prof. Salcedo (ETS Ingenieros Agrónomos, Madrid, Spain) and was tested at a concentration of 20μg/mL.
For in vitro tests, leaves from tobacco were defatted, ground into small pieces and extracted by magnetic stirring in agitation in 50mM phosphate-buffered saline (PBS) at pH 7.5 during 16h at 4°C. The sample was centrifuged at 5600g for 30min and the supernatant was dialysed against water. The dialysed extract was filtered through a 0.22μm-pore diameter membrane and freeze-dried.
Grass pollen extractsExtracts from grass pollens and rye grass pollen (protein concentration 0.5mg/mL) were provided by Bial-Aristegui Laboratories, Bilbao, Spain.
Skin prick testsSkin prick test (SPT) with extracts from tobacco, potato, tomato, mugwort pollen, Cynodon dactylon pollen and Lolium perenne pollen (Bial Aristegui Laboratories, Bilbao, Spain) were performed according to standard procedures,11 over the volar side of the forearm; one sterile device was used for each test. Histamine phosphate (10mg/ml) and sterile 0.9% saline were used as positive and negative controls, respectively. A mean diameter of 3mm or greater than the negative control and a mean area of 7mm2 with hevein, 15min after puncture, was considered a positive response. SPT with all these extracts were also performed in a control group of atopic subjects.
Bronchial challenge testsSpecific bronchial challenge tests (BCT) were carried out following the procedure proposed by Chai et al., with modifications.12 Aerosolised particles generated by a continuous pressurised De Vilbiss 646 nebuliser were inhaled for 2min at normal breathing volume, starting with control PBS solution, and followed - at 10min intervals - by progressive concentrations of the extract. Pulmonary function tests were obtained 30 and 60s after each dose. A positive response was defined as a greater than 20% fall from basal FEV1. After specific BCT, hourly peak expiratory flow (PEF) measurements were recorded for 9 hours. We used an extract of fresh-tobacco leafs with a concentration 1/10 w/v to eliminate other possible additives of cured-tobacco from cigarettes.
Patch testNicotine patch test was performed using commercial nicotine patch (Nicotinell, Novartis Consumer Health S.A.); tobacco patch test was performed with an extract from tobacco leaf 1/10 w/v in vaseline. We also performed a standard series of 20 substances recommended by the European Environmental and Contact Dermatitis Research Group and provided by ALK-Abelló, Madrid Spain, including latex (True tests). Readings were taken at 48, 72 and 96 hours.
Specific IgE measurementsTotal IgE was determined by the CAP System IgE FEIA (Pharmacia Diagnostics, Uppsala, Sweden), following manufacturer’s instructions.
Determination of specific IgE to grass pollens, tobacco, mugwort pollen, tomato and latex was performed by CAP-FEIA technique, following the manufacturer´s instructions (Pharmacia, Uppsala, Sweden) and the results were expressed in kU/L.
Specific IgE to tobacco extract was measured by CAP- Pharmacia technique and EAST technique (Enzyme AllergoSorbent Test). Solid-phase was obtained by coupling the extract solution (10mg/ml) to the 6-mm diameter CNB-activated paper discs as described by Ceska and Lunqvist.13 EAST was performed and results expressed in accordance with the manufacturer´s instructions (Specific IgE EIA kit. HYTEC. HYCOR Biomedical Ltd. UK). EAST results equal to or higher than 0.35kU/L were considered positive (EAST classes from 1 to 4). A pool of sera from non-allergic subjects was used as negative control.
SDS-PAGE ImmunoblotingThe proteins separated by SDS-PAGE were transferred from the polyacrylamide gels onto PVDF membranes (Hybond-P. Amersham Pharmacia Biotech. England) by means of electrophoretic transfer and subsequently incubated with the patient sera. The blot was developed with quimioluminiscence detection system using anti-IgE peroxidase conjugate developed with Lumigen PS-3 (ECL-Plus, Amersham Pharmacia Bitech). Immunoblots were analysed in a GS-710 Image Analyser using the Diversity Database program (Bio Rad Laboratories, Hercules, CA). These assays were also performed using tobacco leaf and latex extracts previously incubated with mercaptoethanol.
Immunobloting- inhibitionThe electro-transferred proteins were incubated with the patient’s sera, which had been previously incubated overnight at 4°C with the corresponding extract (tobacco leaf and latex with and without mercaptoethanol). Patient’s sera used in inhibition assays were previously incubated with tobacco extract (protein concentration 2mg/mL) and latex extracts (protein concentration 2mg/mL).
Statistical analysisData base and statistical analysis was performed using the SPSS for Windows v.11.5 program package (©SPSS Inc., 1989–1999, Chicago, IL, USA). Variables have been described as mean±DS or by frequency and percentage where needed. To compare qualitative variables, the Chi square and the Fisher’s exact test were used. To confirm the normal distribution of variables the Kolmogorov-Smirnov test was used. To compare mean values, the Student’s t-test or the Mann Whitney’s U were performed where needed. Finally, to compare several quantitative variables a two-way ANOVA with a post-hoc Bonferroni test were performed. A p value <0.05 was considered statistically significant.
Informed consentWritten informed consent was obtained from all study participants.
Ethical ApprovalEthical approval was obtained from the Ethical Committee of the Rio Hortega University Hospital.
ResultsClinical data of selected patientsWe randomly selected 15 patients who suffered from sensitisation to latex. All these patients were tested to try to identify tobacco and latex as risk-factors of bronchial inflammation.
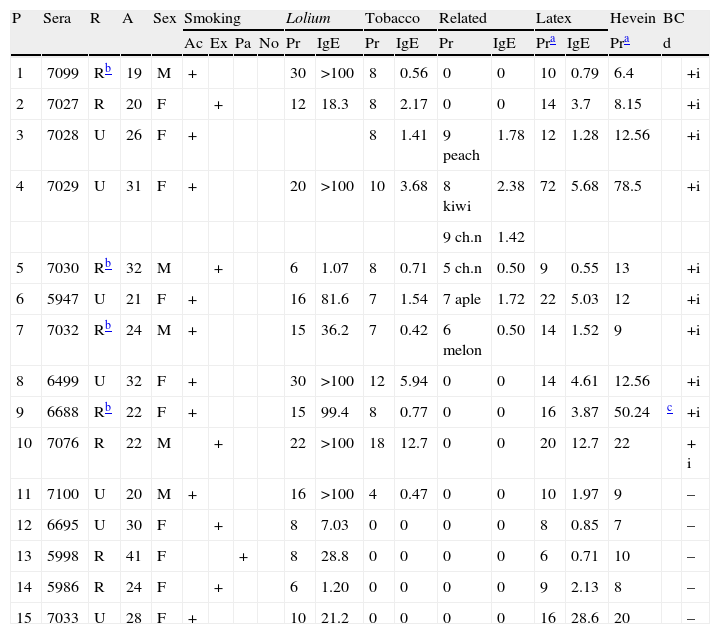
Among the 15 patients sensitised to latex, 5 were male and 10 female (mean age 26.1SD=13.4) (Table 1). All 15 patients showed sensitisation to Lolium perenne (Rye grass pollen) but only patients 1 to 11 had moderate or severe asthma during the pollination period. Patients 12 to 15 were nurses and had very mild symptoms during the pollination period but suffered from asthma when they manipulated latex gloves.
Clinical data of patients
| P | Sera | R | A | Sex | Smoking | Lolium | Tobacco | Related | Latex | Hevein | BC | ||||||||
| Ac | Ex | Pa | No | Pr | IgE | Pr | IgE | Pr | IgE | Pra | IgE | Pra | d | ||||||
| 1 | 7099 | Rb | 19 | M | + | 30 | >100 | 8 | 0.56 | 0 | 0 | 10 | 0.79 | 6.4 | +i | ||||
| 2 | 7027 | R | 20 | F | + | 12 | 18.3 | 8 | 2.17 | 0 | 0 | 14 | 3.7 | 8.15 | +i | ||||
| 3 | 7028 | U | 26 | F | + | 8 | 1.41 | 9 peach | 1.78 | 12 | 1.28 | 12.56 | +i | ||||||
| 4 | 7029 | U | 31 | F | + | 20 | >100 | 10 | 3.68 | 8 kiwi | 2.38 | 72 | 5.68 | 78.5 | +i | ||||
| 9 ch.n | 1.42 | ||||||||||||||||||
| 5 | 7030 | Rb | 32 | M | + | 6 | 1.07 | 8 | 0.71 | 5 ch.n | 0.50 | 9 | 0.55 | 13 | +i | ||||
| 6 | 5947 | U | 21 | F | + | 16 | 81.6 | 7 | 1.54 | 7 aple | 1.72 | 22 | 5.03 | 12 | +i | ||||
| 7 | 7032 | Rb | 24 | M | + | 15 | 36.2 | 7 | 0.42 | 6 melon | 0.50 | 14 | 1.52 | 9 | +i | ||||
| 8 | 6499 | U | 32 | F | + | 30 | >100 | 12 | 5.94 | 0 | 0 | 14 | 4.61 | 12.56 | +i | ||||
| 9 | 6688 | Rb | 22 | F | + | 15 | 99.4 | 8 | 0.77 | 0 | 0 | 16 | 3.87 | 50.24 | c | +i | |||
| 10 | 7076 | R | 22 | M | + | 22 | >100 | 18 | 12.7 | 0 | 0 | 20 | 12.7 | 22 | + i | ||||
| 11 | 7100 | U | 20 | M | + | 16 | >100 | 4 | 0.47 | 0 | 0 | 10 | 1.97 | 9 | – | ||||
| 12 | 6695 | U | 30 | F | + | 8 | 7.03 | 0 | 0 | 0 | 0 | 8 | 0.85 | 7 | – | ||||
| 13 | 5998 | R | 41 | F | + | 8 | 28.8 | 0 | 0 | 0 | 0 | 6 | 0.71 | 10 | – | ||||
| 14 | 5986 | R | 24 | F | + | 6 | 1.20 | 0 | 0 | 0 | 0 | 9 | 2.13 | 8 | – | ||||
| 15 | 7033 | U | 28 | F | + | 10 | 21.2 | 0 | 0 | 0 | 0 | 16 | 28.6 | 20 | – | ||||
All patch tests were negative with tobacco leaf and nicotine except in Patient 66889 who had a delayed response with cured tobacco leaf and negative with nicotine.
Eight patients were smokers; six ex-smokers; and one was a passive smoker. All the patients who had avoided smoking (five females and one male) improved their symptoms after cessation of this habit.
Of these 15 patients, 11 (73.33%) presented sensitisation to tobacco demonstrated by SPT, specific IgE and positive bronchial challenge tests with tobacco extract positive. These patients (1 to 11) presented more severe obstructive parameters in their functional respiratory tests (p<0.001). Of these patients, six came from rural areas and four of them12–15 could have possibly used tobacco as pesticide, but they did not have antecedents of contact with latex except for one woman (patient 9) who occasionally used natural rubber latex gloves for gardening and noted itching and contact-urticaria in her hands. Only this woman presented patch-tests positive to latex and tobacco leaf and delayed response in bronchial challenge with tobacco. Patch tests were negative with tobacco leaf and nicotine in the other 14 patients. None of the patients presented patch tests positive to latex apart from patient 11 who presented contact dermatitis after the use of condoms.
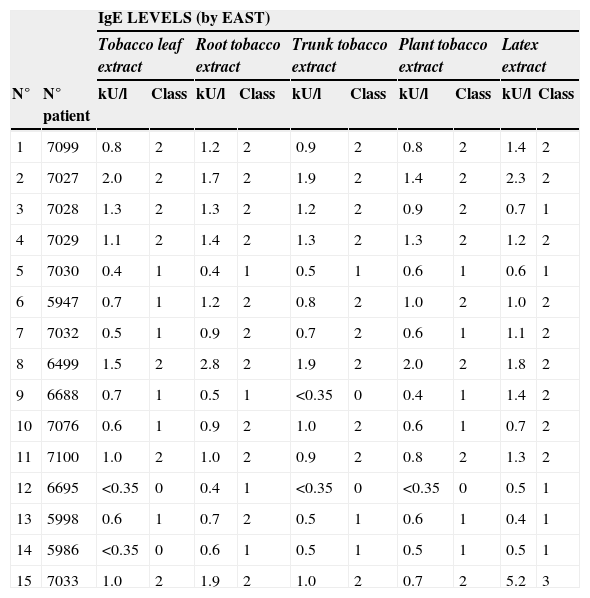
Tobacco IgE level was related with sensitisation to latex (p<0.002) and Lolium perenne (p<0.001), but not with other vegetables that belong to the Solanaceae family. Tobacco IgE level to different sources of the tobacco plant can be seen in Table 2.
IgE levels measured by EAST to different tobacco extracts
| IgE LEVELS (by EAST) | |||||||||||
| Tobacco leaf extract | Root tobacco extract | Trunk tobacco extract | Plant tobacco extract | Latex extract | |||||||
| N° | N° patient | kU/l | Class | kU/l | Class | kU/l | Class | kU/l | Class | kU/l | Class |
| 1 | 7099 | 0.8 | 2 | 1.2 | 2 | 0.9 | 2 | 0.8 | 2 | 1.4 | 2 |
| 2 | 7027 | 2.0 | 2 | 1.7 | 2 | 1.9 | 2 | 1.4 | 2 | 2.3 | 2 |
| 3 | 7028 | 1.3 | 2 | 1.3 | 2 | 1.2 | 2 | 0.9 | 2 | 0.7 | 1 |
| 4 | 7029 | 1.1 | 2 | 1.4 | 2 | 1.3 | 2 | 1.3 | 2 | 1.2 | 2 |
| 5 | 7030 | 0.4 | 1 | 0.4 | 1 | 0.5 | 1 | 0.6 | 1 | 0.6 | 1 |
| 6 | 5947 | 0.7 | 1 | 1.2 | 2 | 0.8 | 2 | 1.0 | 2 | 1.0 | 2 |
| 7 | 7032 | 0.5 | 1 | 0.9 | 2 | 0.7 | 2 | 0.6 | 1 | 1.1 | 2 |
| 8 | 6499 | 1.5 | 2 | 2.8 | 2 | 1.9 | 2 | 2.0 | 2 | 1.8 | 2 |
| 9 | 6688 | 0.7 | 1 | 0.5 | 1 | <0.35 | 0 | 0.4 | 1 | 1.4 | 2 |
| 10 | 7076 | 0.6 | 1 | 0.9 | 2 | 1.0 | 2 | 0.6 | 1 | 0.7 | 2 |
| 11 | 7100 | 1.0 | 2 | 1.0 | 2 | 0.9 | 2 | 0.8 | 2 | 1.3 | 2 |
| 12 | 6695 | <0.35 | 0 | 0.4 | 1 | <0.35 | 0 | <0.35 | 0 | 0.5 | 1 |
| 13 | 5998 | 0.6 | 1 | 0.7 | 2 | 0.5 | 1 | 0.6 | 1 | 0.4 | 1 |
| 14 | 5986 | <0.35 | 0 | 0.6 | 1 | 0.5 | 1 | 0.5 | 1 | 0.5 | 1 |
| 15 | 7033 | 1.0 | 2 | 1.9 | 2 | 1.0 | 2 | 0.7 | 2 | 5.2 | 3 |
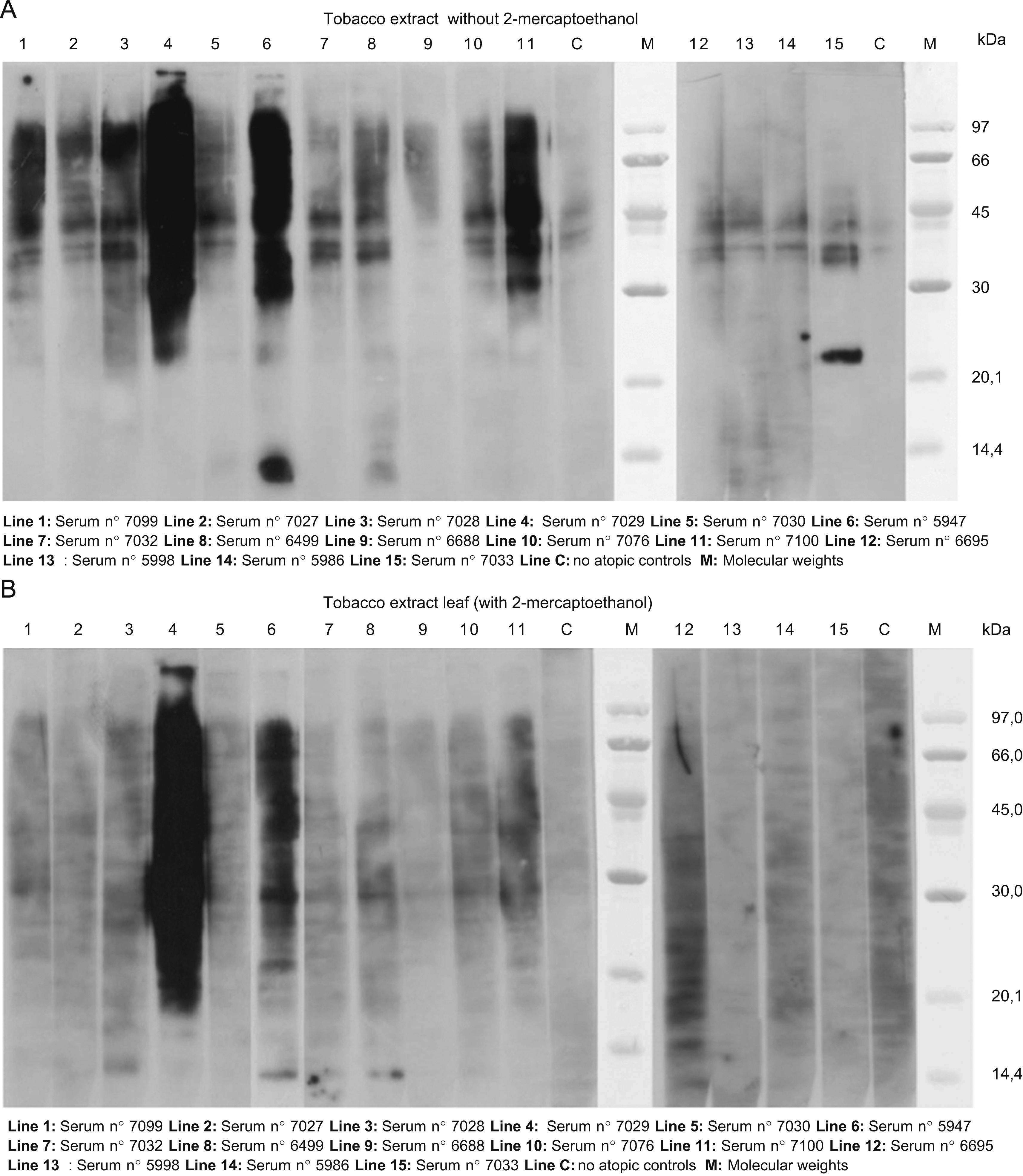
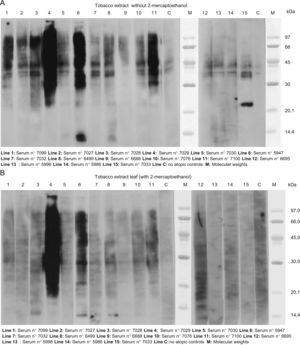
The results of SDS-PAGE Immunoblotting with tobacco leaf can be seen in Figure 1 (A and B). In the assay performed without 2-mercaptoethanol we found a high IgE detection between 30 to 90KDa bands with the sera of almost all the patients. A band of approximately 21KDa was detected with serum 7033 (patient 15) and bands of 13 and 14KDa with sera number 5947 (patient 6) and 6499 (patient 8), respectively.
A. Results of SDS-PAGE Immunoblotting with tobacco leaf. In the assay performed without 2-mercaptoethanol we found a high IgE detection between 30 to 90KDa bands with the sera of almost all the patients. A band of approximately 21KDa was detected with serum 7033 (patient 15) and bands of 13 and 14KDa with sera number 5947 (patient 6) and 6499 (patient 8) respectively. B. The same assay performed with 2-mercaptoehanol. Only a high detection can be seen in sera 7029 and 5947 (patients 1 and 6 respectively). A light detection was observed between 37–45KDa with some sera and a band of 13KDa with sera number 7028, 5947 and 6499; patients 3, 6, and 8, respectively.
In the assay performed with 2-mercaptoehanol only a high detection could be seen in sera 7029 and 5947 (patients 1 and 6 respectively). A light detection was observed between 37–45KDa with some sera and a band of 13KDa with sera numbers 7028, 5947 and 6499; (patients 3,6,and 8 respectively).
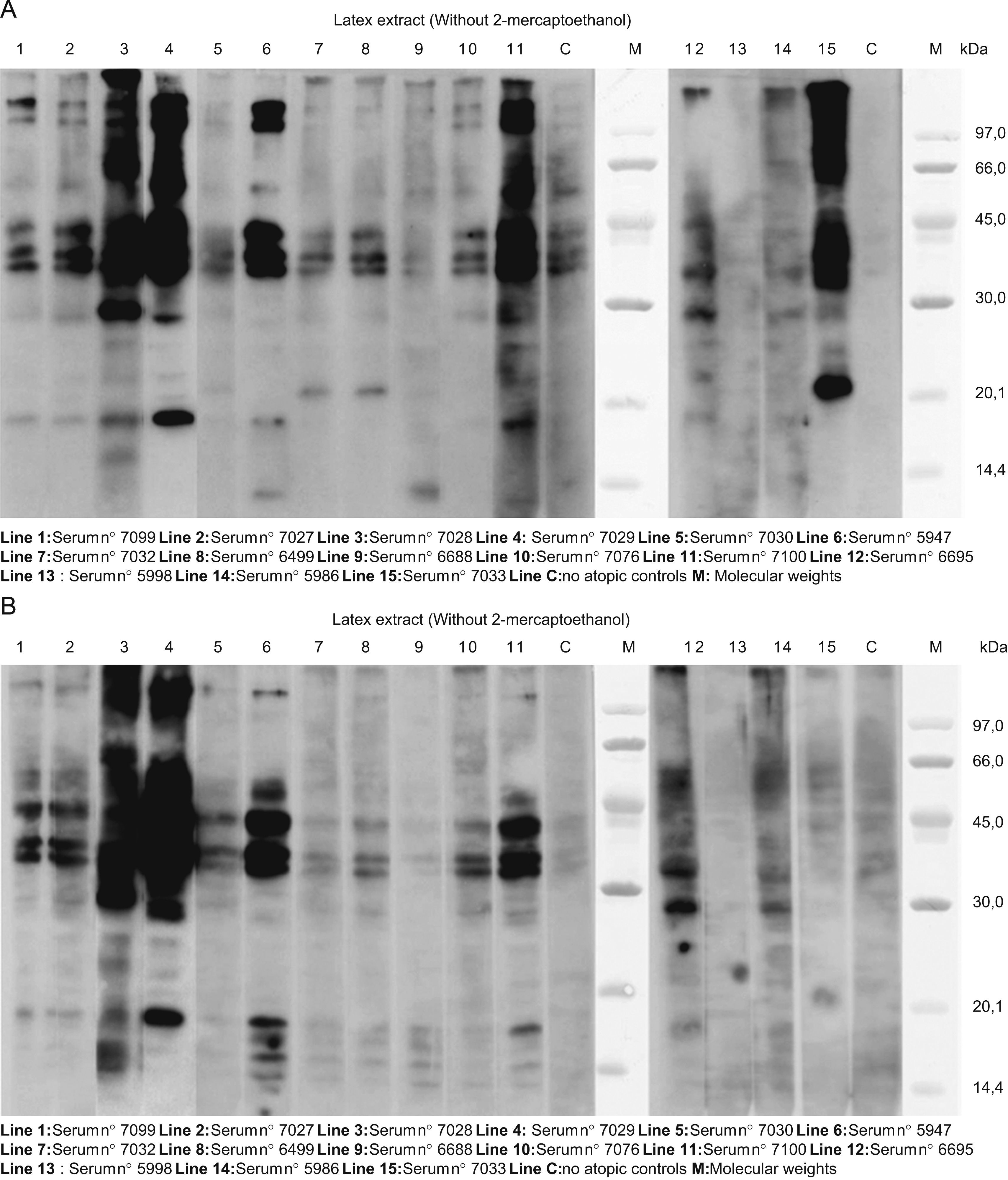
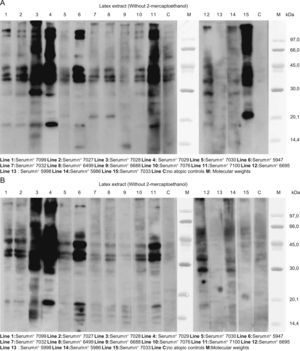
Figure 2 presents the SDS:PAGE immunobloting with latex extracts. Without mercaptoethanol, (Figure 2A), almost all the lines show bands of approximately 132, 109, 58, 44, 40, 37, 29 and 19KDa. In some lines, there were bands of approximately 22 and 14KDa. With mercaptoethanol, (Figure 2B) almost all the sera had similar patterns: 42–36 and 34KDa bands, and 18, 17, 16 and 14.5KDa bands.
A. SDS:PAGE immunoblotting with latex extracts. Without mercaptoethanol, in almost all the lines we can see bands of approximately 132, 109, 58, 44, 40, 37, 29 and 19KDa. In some lines, there were bands of approximately 22 and 14KDa. B. The same assay with mercaptoethanol: almost all the sera had similar pattern: 42–36 and 34KDa bands, and 18, 17, 16, and 14.5KDa bands.
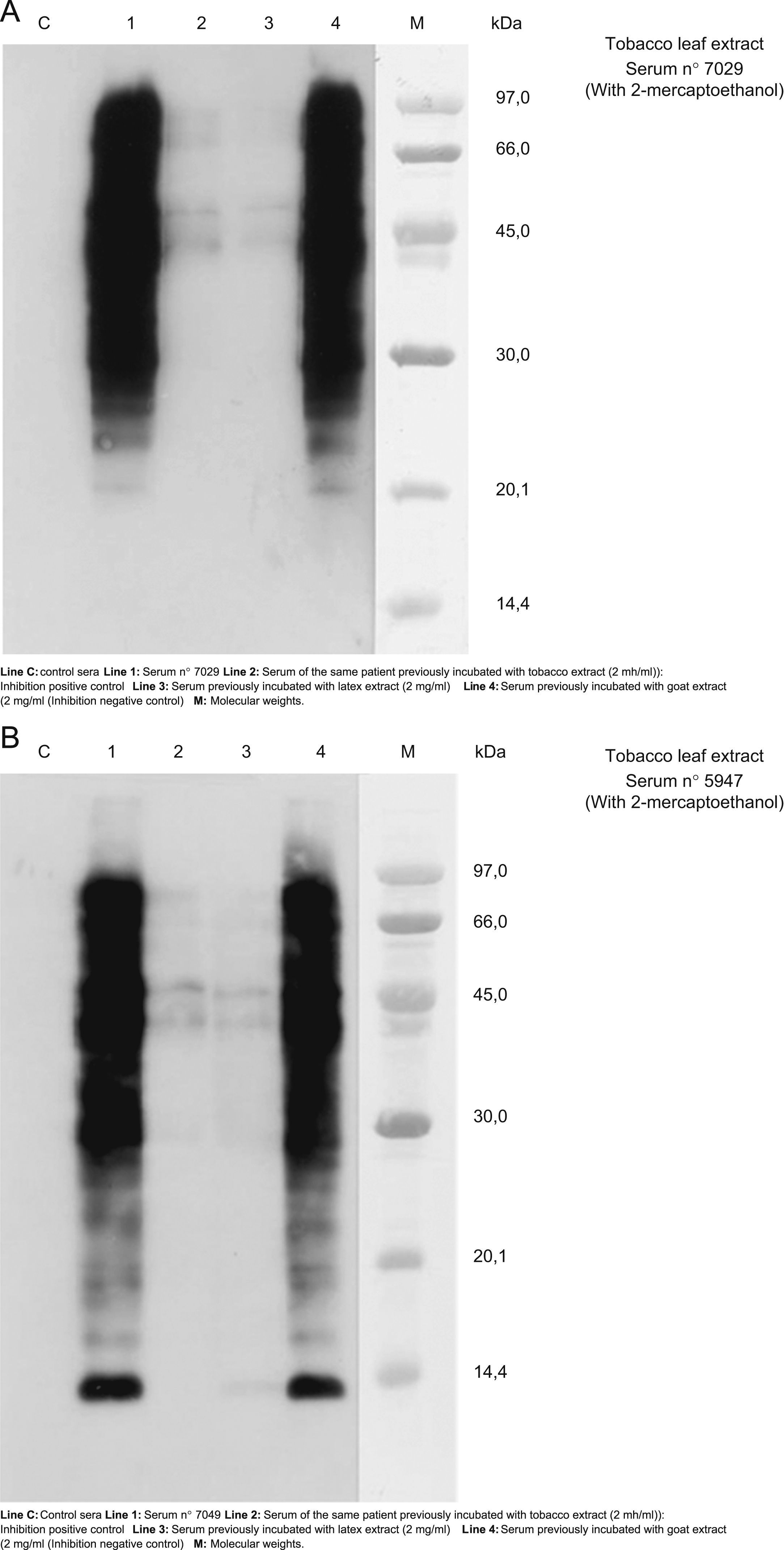
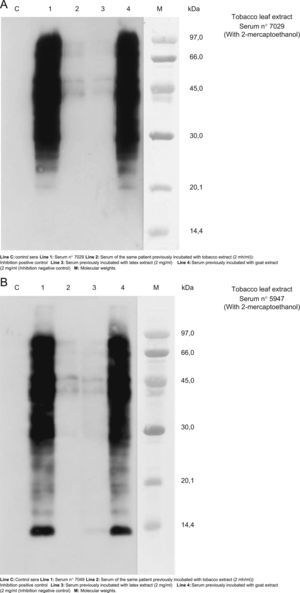
The results of cross-reactivity using SDS-PAE Immunoblotting inhibition (with and without 2-mercaptohetanol) can be seen in Figure 3. We chose the sera number 7029 and 547 (patients 4 and 6 respectively) as representative of other patients because in the blot-assay with these sera all bands of detection of IgE were evident. These results suggest cross-reactivity between tobacco and latex extracts.
A and B. Results of cross-reactivity using SDS-PAE Immunoblotting inhibition using the sera numbers 7029 and 547 (patients 4 and 6 respectively) as representation of other patients because in the blott-assay with these sera all bands of detection of IgE were showed. These results evidenced a cross-reactivity between tobacco and latex extracts.
Patient 12 (sera 6695, line 12 in figures) did not have specific IgE to tobacco leaf, but had levels of specific IgE to root tobacco extract (0.4U/L) and latex (0.5KU/L). Patient 14 did not have specific IgE to tobacco leaf but had levels of IgE to root tobacco extract (0.6KU/L), plant tobacco and latex (0.5KU/L). This suggests that the root and stem of the plant have more concentration of the responsible allergen.
DiscussionThe joint effect of genetic propensity to asthma and exposure to environmental tobacco smoke on the risk of asthma may be greater than expected on the basis of their independent effects.15–22 Nevertheless, while there are studies that suggest that parental smoking may contribute to arise levels of airway responsiveness as early as the first 2 to 10 weeks of age15–17 and that maternal smoking may be associated with an increased incidence of asthma and an earlier onset of disease,18 other studies have demonstrated an association between current exposure to tobacco smoke and a low risk for atopic disorders in smokers themselves and a similar tendency in their children.19
Subjects exposed to cigarette smoke through either active15 or passive routes16 may present an increase in airways responsiveness. The mechanisms by which exposure to environmental tobacco smoke exerts its effect are unknown but may include effects on the IgE immune system that can be elicited both in uterus and after birth.17 Skin testing and RAST have verified the existence of tobacco-specific IgE in some studies.8,10,23 However, the very few published studies report conflicting results concerning the clinical significance of tobacco IgE.
There are different studies that have shown a modification of the immune-response caused by tobacco.24–30 In our previous preliminary report a possible clinical significance of tobacco IgE sensitisation in the development of asthma was suggested;10 and our results in this new study show that patients with asthma due to pollen and also sensitised to latex are the people with more positive clinical responses to tobacco, possibly due to a cross-reactivity between latex and tobacco allergens. Patients sensitised to latex in their work (nurses) had not clinical symptoms due to pollen, and bronchial challenge tests with tobacco were negative. Nevertheless, when sensitisation was firstly due to pollen, positive bronchial challenges with tobacco were obtained, and asthma symptoms were more severe.
Tobacco habit can modify the inflammatory response associated with asthma by reducing the number of eosinophils,26 and also the IL-18 concentration in sputum,27 modifying the balance of cytokines Th1/Th2,28 increasing oxidative stress29 and producing airways remodelling.30,31 Nevertheless the possibility of allergic reactions to tobacco allergens has been underestimated. Smoker patients are exposed everyday to inhalation of tobacco particles when handling cigarettes. Tobacco habit is also a possible risk factor for occupational asthma,30 as is latex. Tobacco, cocoa, coffee and ragweed are cross-reacting allergens that can activate factor XII-dependent pathways.32 In the case of tobacco, the activation of the classical pathway of complement is by tobacco glycoprotein (TGP).33 Vegetal glycoproteins have been implicated in vegetal defence and also as potential panallergens.34–39 Prohevein-like defence protein of tobacco is a cross-reactive allergen for latex-allergic patients.6 Tobacco contains a 21-Kd prohevein-like allergen (CBP29LP) which is targeted by IgE to the majority of the NRL-allergic patient sera as can be seen in immunoblotting results. All our rye-grass sensitised patients recognised bands around 30–45KDa, the same molecular weight range to defence proteins from rye grass pollen40–44.
In summary, we suggested that the association of sensitisation to latex and tobacco seems to strength the severity of asthma symptoms in patients also sensitised to pollen. Possible cross-reactivity between latex and tobacco allergens seems to exist. Smoker patients with IgE response to tobacco might be a risk population for latex sensitisation.
Conflict of interestWe do not have any financial relationship with a biotechnology and/or pharmaceutical manufacturer that has an interest in the subject matter or materials discussed in the submitted manuscript.
The authors are grateful to K. Adams for language advice. This work was supported by SACYL, grant 05.02.467B01.144312. Project in biomedicine entitled “Allergy hypersensitivity to cannabis and tobacco in allergic patients”.