Papular urticaria caused by flea bite presents clinical symptoms of a hypersensitivity reaction accompanied by skin lesions. However, the pattern of recognition by different antibody isotypes during the progression of the disease is unknown. This study evaluated variations in immunoglobulin E and immunoglobulin G subclass antibody responses to flea antigens during the progression of papular urticaria caused by flea bite
MethodsTwenty-five patients clinically diagnosed with papular urticaria due to flea bite were included. Ten healthy children were included as controls. Recognition of antigens from complete flea body extract by patients and healthy controls was determined using immunoblot assays.
ResultsThe results revealed that patients with 2–5 years of papular urticaria evidenced more IgE bands than those with shorter or longer durations of symptoms. In contrast, healthy children showed a predominance of immunoglobulin G1 and immunoglobulin G3. The majority of the recognised antigens were low molecular weight proteins (<90kDa). Proteins with molecular weights between 16–20, 21–25, and 31–35kDa showed different patterns of recognition between patients and healthy children.
ConclusionThe predominant specific antibody isotypes vary according to the time elapsed since the onset of symptoms in papular urticaria caused by flea bite.
Papular urticaria is a pathology caused by the bite of insects such as mosquitoes, bedbugs, flies and fleas, among others.
Papular urticaria caused by flea bite (PUFB) is a hypersensitivity reaction characterised by groups or clusters of papules, frequently associated with grouped itchy papules. The papules are often accompanied by intermittent and chronic excoriation that results in hypo- or hyper-pigmented macules, severe infections, and scars. The pathophysiology of papular urticaria remains to be fully elucidated. The presence of eosinophils, the predominance of CD4 T lymphocytes, and the observed immunoglobulin (Ig) E and IgG response patterns indicate that the immune reaction to flea antigens in human patients may occur through more than one mechanism.1
The role of antibodies has been demonstrated in the development of allergic inflammation mediated by IgE and IgG subclasses, by activating mast cells and the formation of immune complexes.
Regarding the humoral immune response associated with PUFB, canine models have shown that dogs with hypersensitivity to flea bites have higher levels of specific IgE and IgG antibodies to flea antigens than dogs that have never been bitten by fleas.2 Additionally, a human study demonstrated IgE and IgG recognition of flea extract proteins within a range of 26–150kilodaltons (kDa). However, in this study, recognition of flea proteins by IgE tends to decrease with disease progression.1
In papular urticaria by mosquito bite, elevated mosquito saliva serum-specific IgE has been detected in mosquito-allergic or mosquito bite test positive subjects. The time frame of the immediate reaction to mosquito bites is compatible with that of IgE-mediated type I hypersensitivity. Mosquito bite-induced immediate wheals and flares correlate well with mosquito salivary gland-specific IgE levels. The development of skin sensitisation to mosquito bites also parallels the levels of saliva specific IgE antibodies. In individuals with systemic reactions, saliva-specific IgE levels are significantly increased. Mosquito saliva-specific IgG antibodies, consisting mainly of the IgG4 and IgG1 subclasses, have been found to be significantly elevated in individuals with positive mosquito bite tests and in individuals with severe local reactions, but not systemic reactions, to mosquito bites. Levels of mosquito saliva-specific IgG correlate with the sizes of both immediate and delayed skin reactions and with saliva-specific IgE levels.3
To date, it is not clear whether there is a differential isotype pattern associated with PUFB. The aim of the present study was to identify variations in IgE and IgG subclass-mediated responses to flea antigens during the progression of PUFB.
Materials and methodsStudy populationThe sample included 25 patients, aged 1 to 15 years, who were clinically diagnosed with PUFB. They were attended to by the Pediatric Dermatology and Allergy division of the Fundación Santa Fe de Bogotá, in Bogotá, Colombia. Patients excluded from consideration for participation in the study included those with secondary infected lesions, immuno-suppression due to systemic disease, treatment with immuno-suppressive medication five days before the consultation, and/or treatment with flea extract. Ten healthy children admitted to the same institution for elective surgery (hernia or circumcision) within the same age-group, and sharing the same socioeconomic characteristics as PUFB patients, without evidence of significant differences for this study because they did not present antecedents of the disease, according to clinical history, were included as controls. This investigation was approved of by the ethics committees at both the Fundación Santa Fe de Bogotá and the Pontificia Universidad Javeriana. Informed consent was obtained from all research participants.
Disease diagnosisThe diagnosis of PUFB was conducted according to clinical characteristics. Patients usually had lesions appearing as grouped papules, a symptom commonly associated with pruritus. Papules were often excoriated or crusted, appearing intermittently in a persistent chronic manner, and leaving behind hypo- or hyper-pigmented macules. They were most frequently located in areas where clothing fits snugly, such as the sock line and waist band. However, exposed areas of the extremities were also affected in some patients.
Obtaining flea antigensA complete flea aqueous extract (10% W/V) was prepared by maceration of Ctenocephalides felis (Greer Laboratories, Lenoir, NC, USA) in PBS with constant shaking for 2 hours at room temperature. The preparation was centrifuged at 390g for 15 min at 4°C and filtered through a 0.22μm pore size membrane. The concentration of the protein was determined by Bradford’s technique. Finally, the extract was aliquoted and stored at −70°C.
Immunoblot analysisIgE and IgG-subclass antibodies were detected by immunoblot analysis.4 Following separation by SDS-PAGE, flea extract proteins were transferred to a nitrocellulose membrane (0.45μm pore size). The immunoreactivity of diluted sera samples was assessed with mouse anti-human IgE and mouse anti-human IgG subclass conjugates labelled with peroxidase and revealed by a chemiluminescent substrate. Each band was considered as positive evidence of antibody recognition of the corresponding antigen.
Considering flea bite exposure in the Bogotá population, reference background levels of flea specific antibodies were established using different dilutions of sera from healthy infants living in areas where climate conditions do not favour the existence of fleas.
Statistical analysisFor data analysis, we used SPSS statistic software. All categorical variables were described using frequencies, while continuous variables were reported as means or medians with corresponding 95% confidence intervals and standard deviations. Comparisons were conducted using the U Mann-Whitney test. To assess differences between IgE and IgG-subclass antibodies against flea antigens in both groups, we used the Fisher exact test. The significance level was set at <0.05.
ResultsIn agreement with our previous studies, in which we find differences in the answer of antibodies IgE and IgG against the flea antigens,1 the patients were subdivided into three groups according to the time elapsed from the initial onset of symptoms: group 1 (2 years since the onset of symptoms, n=10 patients); group 2 (2–5 years since the onset of symptoms, n=10 patients); and group 3 (more than 5 years since the onset of symptoms, n=5 patients). Among all patients included in the study, 28% of the children reported a personal history of atopy (asthma, allergic rhinitis or atopic dermatitis), while 72% reported a familial history of atopy. Among healthy children, none reported a personal history of atopy, and 30% reported a familial history of atopy.
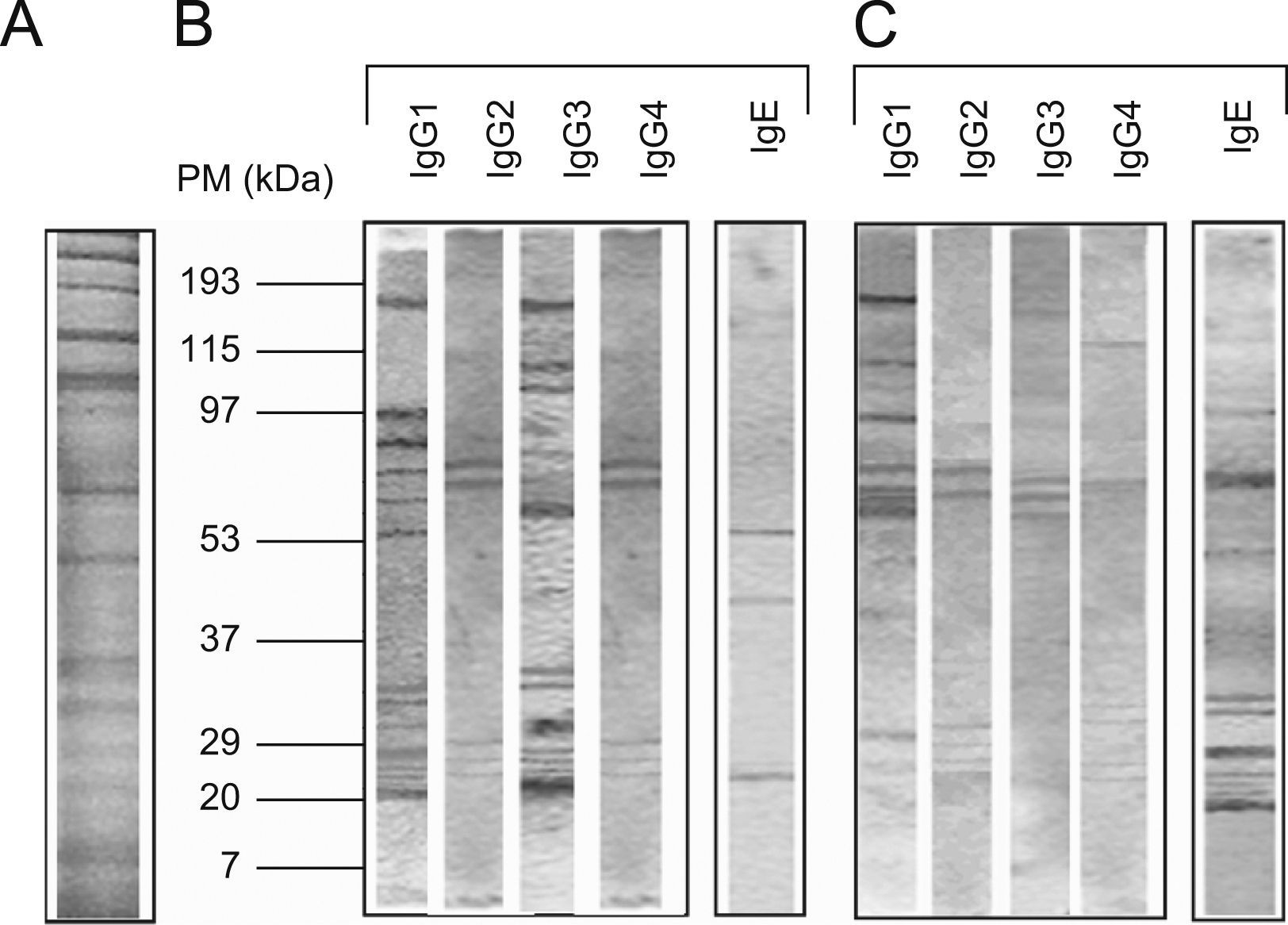
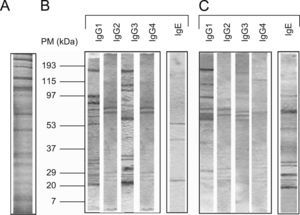
The protein concentration of the flea extract was 1.3mg/ml. According to the pattern of molecular weights, SDS-PAGE revealed ∼30 proteins in the flea extract exhibiting a wide range of molecular weights between 12 and 200kDa (Figure 1). When comparing these results to the analysis of protein recognition by subject antibodies, the majority of the recognised proteins had molecular weights of less than 90kDa. In all cases, we observed a low frequency of recognised proteins with high molecular weights (Figure 1).
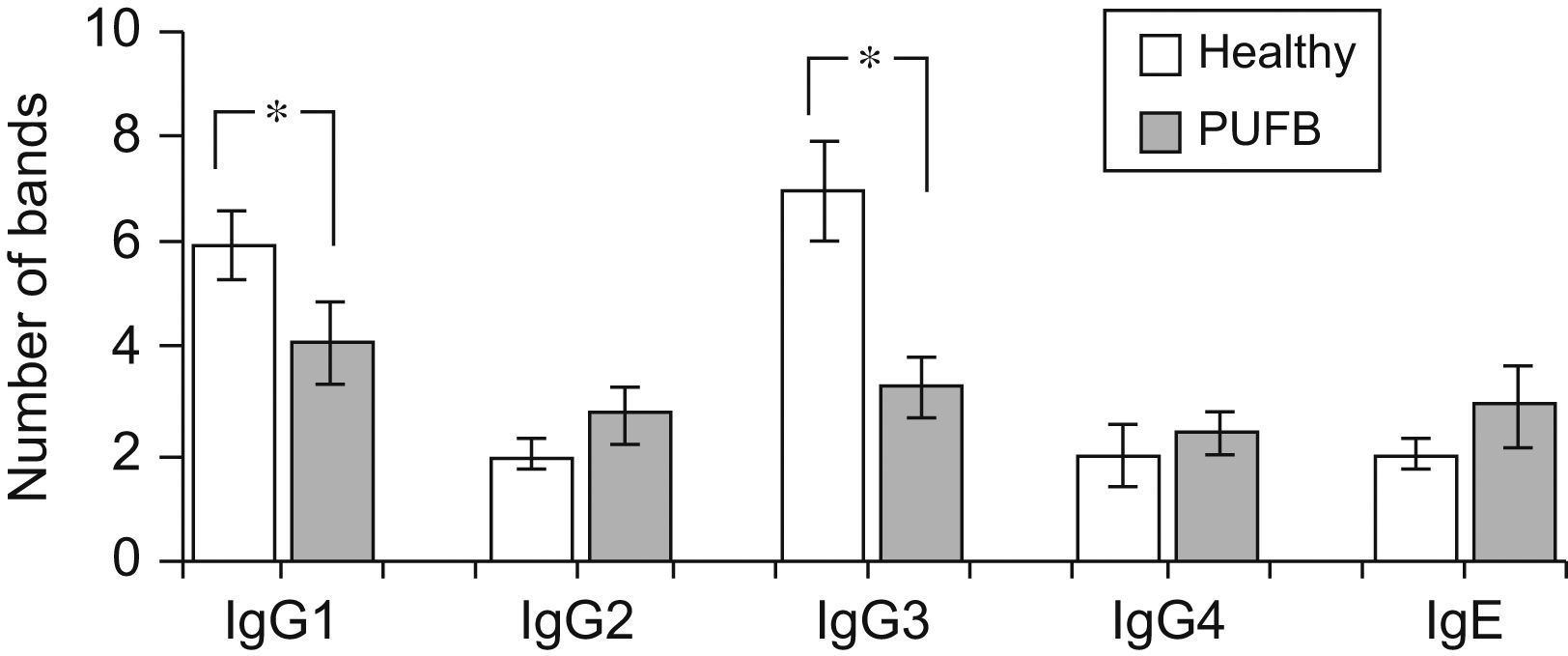
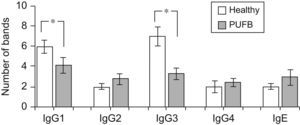
The number of protein bands recognised by each antibody isotype from the sera showed that IgG1 and particularly IgG3 from healthy children recognised a greater proportion of proteins compared to patients with PUFB (p=0.030, p=0.005, respectively) (Figure 2).
Recognition of flea antigens by patients with PUFB and healthy patients. Number of protein bands recognised by antibody isotypes comparing healthy children and patients with PUFB. Results are presented as the mean number of recognised bands with the corresponding standard error. *: p<0.05
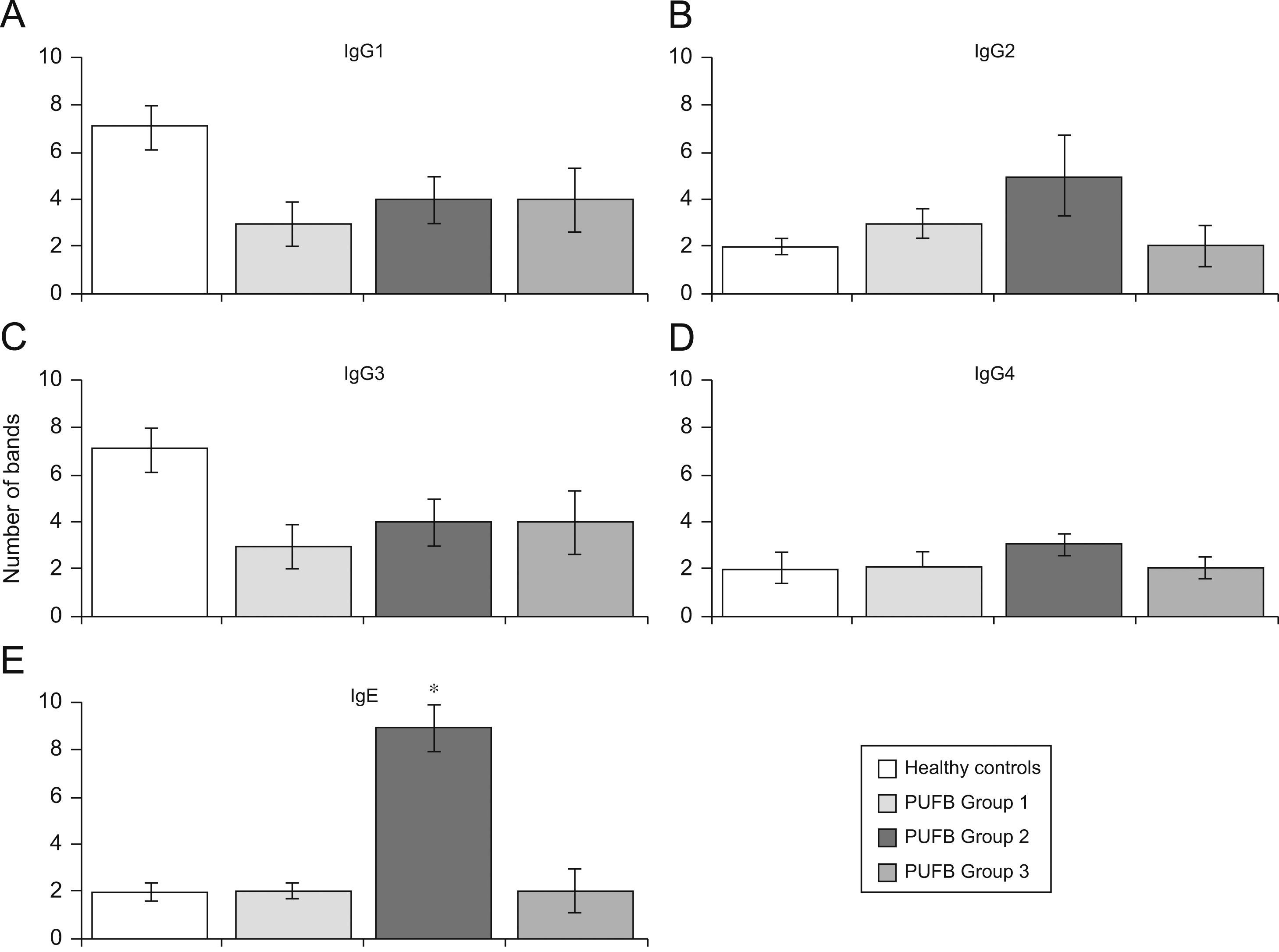
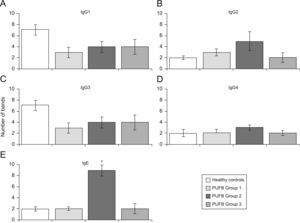
Analysis by groups showed that sera from patients in group 2 (2–5 years of onset) evidenced more IgE bands than those with shorter or longer durations of symptoms (p=0.002) (Figure 3E). No significant differences were found with regard to recognition of flea antigens by IgG2 and IgG4 isotypes.
Recognition of flea antigens by groups of patients with PUFB and healthy patients. Number of protein bands recognised by antibody isotypes comparing healthy children and patients with PUFB subdivided into 3 groups according to the time elapsed since the onset of symptoms. Results are presented as the mean number of recognised bands with the corresponding standard error. *: p=0.002
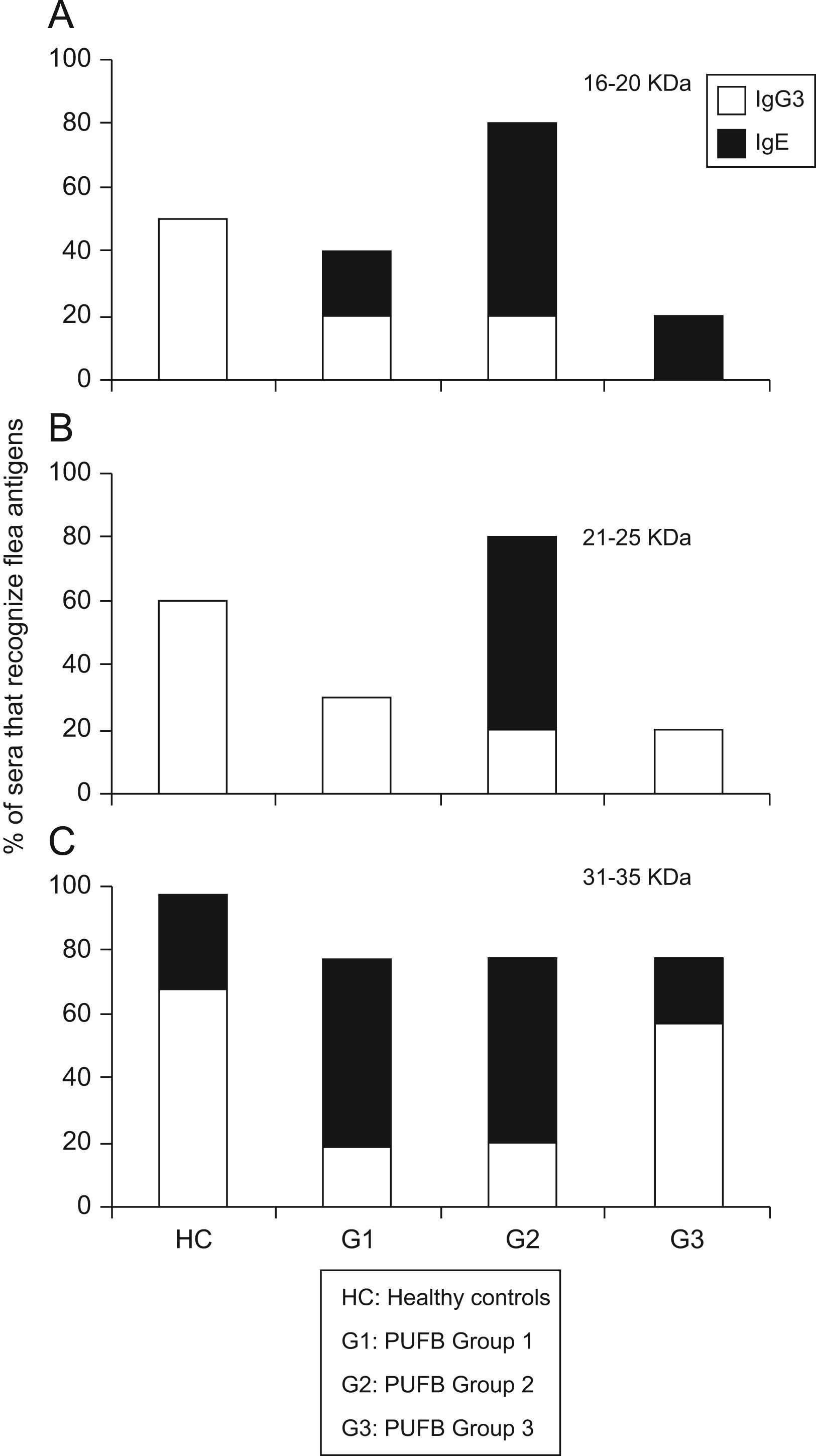
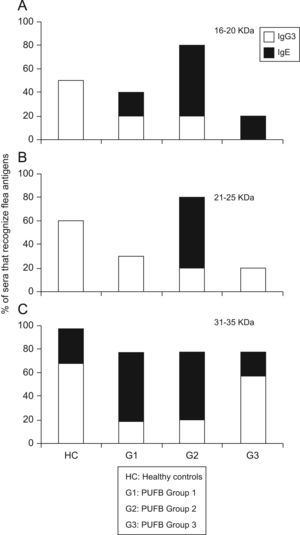
The comparison between proteins recognised by the IgE and IgG subclass showed that those with molecular weights between 16 and 20kDa were recognised in greater proportion by IgG3 (p<0.01) from healthy children, and by IgE from patients within group 2 (p=0.03). The same pattern of recognition was found for proteins with molecular weights between 21 and 25kDa. For proteins between 31 and 35kDa, we observed a significant difference (p=0.03) in recognition by IgG3 and IgE between healthy children and patients in the three groups. Finally, there was greater recognition by IgG3 in healthy children and patients in group 3, whereas patients within groups 1 and 2 showed greater recognition by IgE (Figure 4).
Differential recognition of flea antigens by antibody isotypes. Percentage of sera from patients demonstrating recognition of protein bands with molecular weights between 31–35kDa by IgG3 and IgE isotypes subdivided into 3 groups according to the time elapsed since the onset of symptoms. *: p<0.05
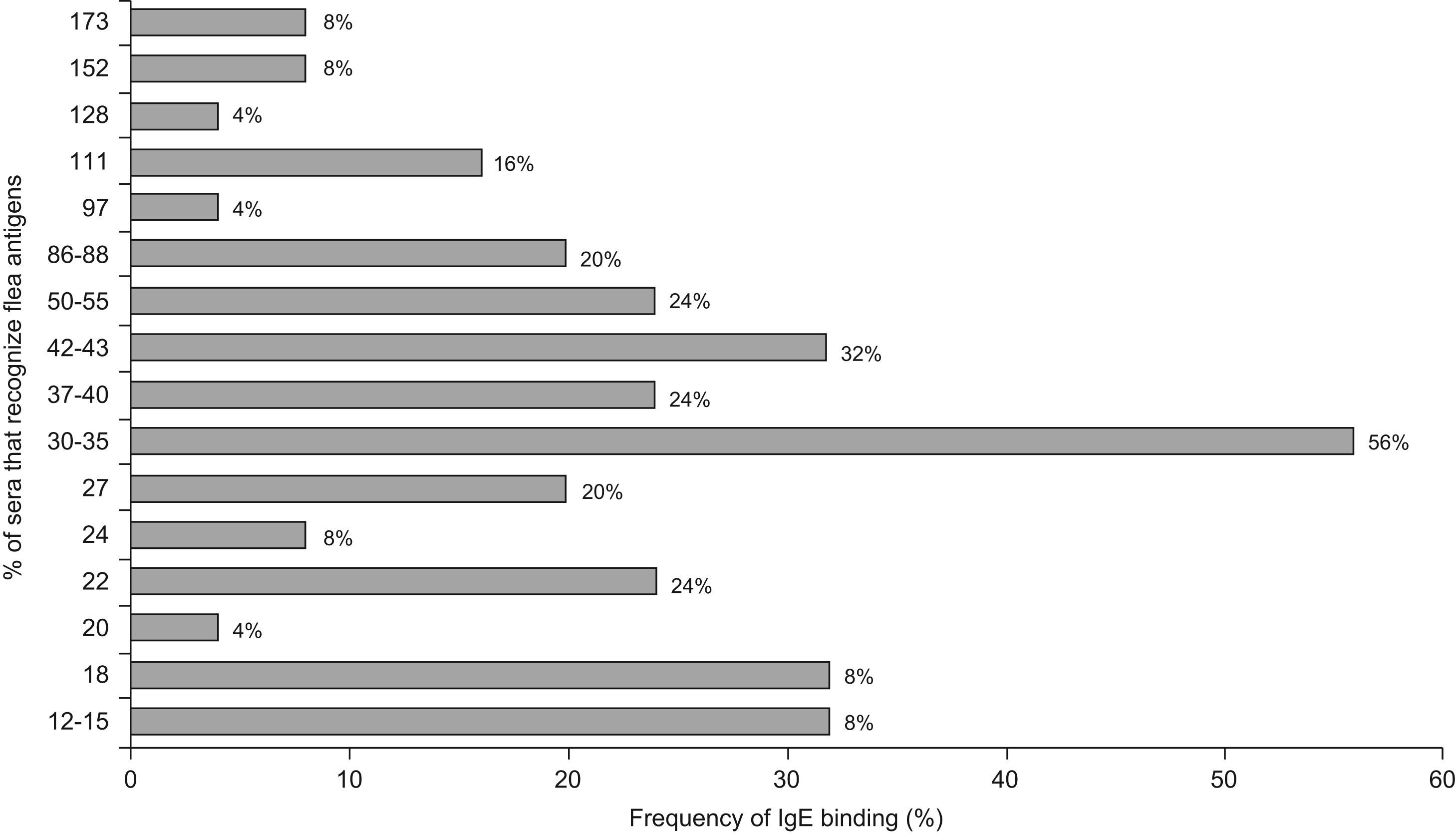
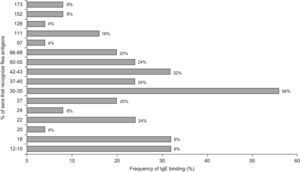
Regarding IgE, the most frequently detected allergens were the 30–35kDa with a reactivity of 56%, 42–43kDa (32%), 50–55kDa, 22kDa and 37–40kDa (24%); 86–88kDa and 27kDa (20%). The remaining allergens were detected with frequencies between 4% and 16% (Figure 5)
DiscussionThe immune response associated with papular urticaria in humans and caused by ectoparasite bites has been mostly studied for mosquitoes.3 Some reports in adult patients with histories of systemic allergic reactions to mosquito bites showed higher levels of IgE antibodies against allergens of different mosquito species compared to healthy adults, although no difference was observed regarding IgG levels.5 When assessing the antibody response in allergic individuals, an increase in IgE, IgG4, and IgG1 during mosquito season was found when compared to the previous season.6
In a previous human study conducted by our research group in which antigenic and allergenic proteins were characterised from complete flea extract, we found that a large number of proteins with a wide range of molecular weights were recognised by both IgE and IgG from patients and controls. Among the recognised proteins, those between 33 and 35kDa demand particular attention as they were recognised by IgE in nearly 60% of both group 1 and group 2 patients.1 The recognition pattern of IgG-subclass from patients with PUFB is unknown, in contrast to the pattern observed in papular urticaria caused by mosquito bite, which has been more extensively studied.
In this research, we also observed recognition of proteins with a wide range of molecular weights. The majority of the recognised proteins in patients, as well as in controls, were less than 90kDa. Common to other allergic diseases, it has been found that antigens considered as major allergens and recognised by a high percentage of sera from allergic patients are of low molecular weight.7–10
The fact that the analysis of protein recognition did not show the same results for all patients (Figure 2) may suggest that the mechanisms that regulate specific humoral immunity are not the same in healthy individuals and patients with PUFB. Furthermore, analysis of protein recognition by each isotype from groups of patients according to the time elapsed from the initial onset of symptoms (Figure 3) suggest that there are differences in recognition that may be related to the progress of the disease.
The analysis of proteins with different molecular weights demonstrated differences, particularly in three groups: 16–20, 21–25, and 31–35kDa, as described in other models. An 18kDa protein isolated from the saliva of the cat flea, Ctenocephalides felis, elicits a positive intradermal skin test in 100 and 80% of experimental and clinical flea allergic dogs, respectively.11 Previous studies examining allergy to mosquito bite have reported proteins with molecular weights similar to those found in the present study (low molecular weights of 68kDa,12 37kDa,13 and 30kDa14) that are recognised by IgE in allergic individuals.
Our results show that for the three groups of proteins mentioned above, there was a significant difference in recognition between IgG3 and IgE isotypes as related to Th1-type and Th2-type cytokine patterns, respectively. Proteins with molecular weights between 31 and 35kDa were of special interest because they showed a similar recognition pattern in patients from group 3 and healthy children. This may indicate that this group of patients is acquiring a natural desensitisation to flea antigens. In agreement with our results, a negative correlation between age and IgE and IgG antibody levels was found in children 1 month to 18 years of age who were allergic to mosquito bites. These levels gradually decreased after 5 years of age, suggesting a natural desensitisation.15
In conclusion, there may be a dynamic evolution of the humoral immune response to flea bite that varies according to the time elapsed since the onset of symptoms.
These results identified the relevant proteins in the allergic response in patients with PUFB. Utilisation of molecular biologic techniques to produce pure flea allergens will make the diagnosis and treatment of PUFB more accurate and safe, and will make the development of innovative allergen immunotherapy possible.
Therapeutic immune intervention strategies based on these findings might contribute to a faster acquirement of immune tolerance to flea antigen in susceptible individuals and, consequently, improve the quality of life of the paediatric population.
The authors wish to thank the collaboration of doctors Manuel Forero, Mariela Tavera, Clara Ordoñez, Ana María Salazar, and Adriana Motta for help to get the patients, and Claudia Satizabal and Juliana Quintero, from Fundación Santa Fe de Bogotá, who contributed to the statistical analyses and edition of the document.