Aspirin-induced asthma (AIA), is a distinct clinical syndrome affecting some asthmatic patients. Although the name of the condition relates to aspirin, it is well established that affected patients are cross-sensitive to all non-steroidal anti-inflammatory drugs (NSAIDs).
ObjectivesAssessing the prevalence of AIA among Saudian asthmatic patients.
MethodsThis is a retrospective study of the medical records of asthmatic patients.
ResultsThe prevalence of AIA in the patients studied was found to be 12.6%. Statistical analyses indicated that AIA was associated with more severe asthma [odds ratio (OR) (95% Confidence interval (CI)) in Group I cases 2.86 (1.24 to 6.59) respectively (p<0.05)]. The results showed that some allergic conditions were significantly more common in Group I for allergic rhinitis (OR=2.19, 95% CI 0.89–5.37, p<0.05), pollinosis (OR=1.59, 95% CI 0.85–2.98, p<0.05) for antibiotic allergy (OR=1.25, 95% CI 0.65–2.41, p<0.05) and for atopic dermatitis (OR=1.34, 95% CI 0.70–2.55, p<0.05). Family history of allergy had a more significant role in Group I cases (OR=1.27, 95% CI 0.68–2.37, p<0.05). No gender difference on asthma severity was detected in either group (Chi2=2.19, p>0.05).
ConclusionThe prevalence of respiratory symptoms triggered by aspirin/NSAID use was 12.6% in the asthmatics studied. AIA appears to be a significant problem and further investigations of the mechanisms of these responses and the possible link between this syndrome and other allergic co-morbidities are required.
Aspirin-induced asthma (AIA) is a clinically distinct syndrome characterised by the precipitation of asthmatic attacks following the ingestion of aspirin (ASA) and other non-steroidal anti-inflammatory drugs (NSAIDs).1 It is more common in women, and in most cases, the clear history should enable the physician to make a diagnosis.2 Despite a wealth of literature on AIA, controversy remains as to its prevalence. Differences in populations studied, methods used, definitions of outcomes, and criteria for defining sensitivity reactions may all be responsible for the variations in reported rates.3–5
Estimation of the prevalence of AIA sensitivity in other studies varied from 3% to 19%, depending on whether the diagnosis was based on history alone or on challenge tests with AIA, or whether the study population consisted of severe hospitalised asthmatics.6 Analyses based on the use of a questionnaire revealed a wide variation of results; however, they were always higher than those obtained from retrospective analyses of medical records. Prevalence rates of 4.3–24% were given in the six studies using questionnaires,5,7–11 whereas rates of 2–3% were obtained from the three studies relying on medical records.12,13
It would appear that AIA is still under-diagnosed. This might possibly be the result of the fact that patients experiencing undesirable reactions after aspirin ingestion tend to avoid taking drugs of the same kind next time round,7 or it might be due to the lack of routine oral/inhaled provocation tests in everyday clinical practice.14,15 The main indication for the challenge is the lack of a clear history of aspirin tolerance or a suspected intolerance. But in patients with a positive history of AIA sensitisation, there is general agreement that the challenge should be done for research purposes or for desensitisation.16
As there is no published data describing the prevalence of AIA in Saudi Arabia, thus the primary aim of this study was to assess the prevalence and characteristics of AIA using retrospective medical records of asthmatic Saudian population to determine the presence of an association with asthma severity, familial history of allergy, and allergic co-morbidities.
Patients and methodsStudy design and populationThis retrospective study was carried out by reviewing medical records for prevalence of AIA in clinically diagnosed Saudian asthmatic patients according to guidelines,17, from the period from March 2008 to April 2009 and in accordance with the ethical principles of the Declaration of Helsinki and Geneva Declaration of the World Medical Association. The source of data was the Allergy Unit in Taif University. The examination of medical records was done by the Chief Consultant of the Unit.
The medical sheet used in the Unit included questions regarding: (1) family history of allergy, (2) associated allergic co-morbidities, (3) risk and provoking factors of asthma and asthma exacerbations including aspirin and NSAIDs (patients were asked specifically about the symptoms precipitated, such as dyspnoea, wheezing, nasal blockage, skin eruption, loss of consciousness; further questions determined time of the reaction and its possible recurrence on the second exposure to the drug). The asthmatics suffering from respiratory symptoms following aspirin or/and NSAIDs ingestion were classified as Group I with Group II representing the rest of the studied population.
Outcome measuresThe primary end points of this study were:- 1.
Determination of the prevalence of AIA in studied Saudian asthmatic patients with any characteristic association with asthma severity, certain gender or familial history of allergy.
- 2.
Detection of a link with other allergic diseases more prevalent in those with AIA than in other asthmatics, as the scientific design of this study focused on identifying and/or evaluating inter-relationships among various medical variables
Statistical analyses were done using the computer-based analysis program SPSS version 15.0 (Chicago, USA). Statistical significance was defined as a p-value<0.05. Prevalence rates of allergic diseases under investigation, as well as odds ratio and 95% confidence intervals were calculated. The comparisons of some indices and other variables in the selected sub-groups were carried out by Chi-square test for independent variables.
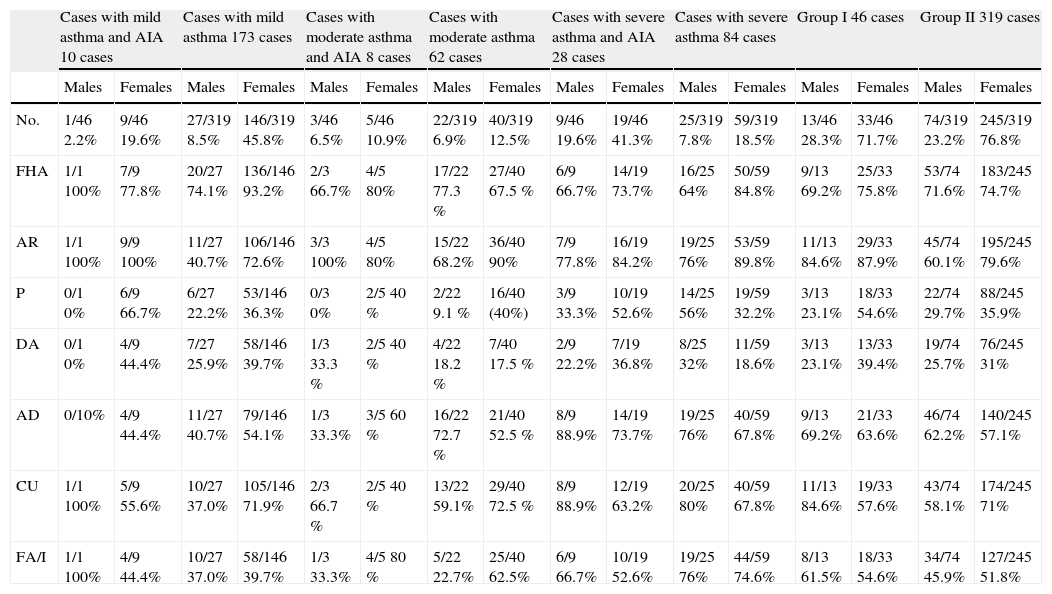
ResultsMedical records of 365 asthmatics (age range 21–56 years) were examined under scrutiny for AIA. The prevalence of AIA in the study population was estimated at 12.6% (46 from a total of 365 cases studied), comprising 33/46 females (71.7%) and 13/46 (28.3%) males. These cases reported respiratory reactions in the form of dyspnoea and wheezing in addition to rhinorrhea after ingesting ASA (22%) or other NSAIDs (78%). No skin reaction was ever recorded. Cases were divided into Group I (46 cases) evidencing AIA and Group II (319 cases) representing the other studied asthmatic population.
Table 1 shows the baseline characteristics (number and percentage) of asthmatics enrolled in this study.
Characteristics of patients enrolled in study
| Cases with mild asthma and AIA 10 cases | Cases with mild asthma 173 cases | Cases with moderate asthma and AIA 8 cases | Cases with moderate asthma 62 cases | Cases with severe asthma and AIA 28 cases | Cases with severe asthma 84 cases | Group I 46 cases | Group II 319 cases | |||||||||
| Males | Females | Males | Females | Males | Females | Males | Females | Males | Females | Males | Females | Males | Females | Males | Females | |
| No. | 1/46 2.2% | 9/46 19.6% | 27/319 8.5% | 146/319 45.8% | 3/46 6.5% | 5/46 10.9% | 22/319 6.9% | 40/319 12.5% | 9/46 19.6% | 19/46 41.3% | 25/319 7.8% | 59/319 18.5% | 13/46 28.3% | 33/46 71.7% | 74/319 23.2% | 245/319 76.8% |
| FHA | 1/1 100% | 7/9 77.8% | 20/27 74.1% | 136/146 93.2% | 2/3 66.7% | 4/5 80% | 17/22 77.3 % | 27/40 67.5 % | 6/9 66.7% | 14/19 73.7% | 16/25 64% | 50/59 84.8% | 9/13 69.2% | 25/33 75.8% | 53/74 71.6% | 183/245 74.7% |
| AR | 1/1 100% | 9/9 100% | 11/27 40.7% | 106/146 72.6% | 3/3 100% | 4/5 80% | 15/22 68.2% | 36/40 90% | 7/9 77.8% | 16/19 84.2% | 19/25 76% | 53/59 89.8% | 11/13 84.6% | 29/33 87.9% | 45/74 60.1% | 195/245 79.6% |
| P | 0/1 0% | 6/9 66.7% | 6/27 22.2% | 53/146 36.3% | 0/3 0% | 2/5 40 % | 2/22 9.1 % | 16/40 (40%) | 3/9 33.3% | 10/19 52.6% | 14/25 56% | 19/59 32.2% | 3/13 23.1% | 18/33 54.6% | 22/74 29.7% | 88/245 35.9% |
| DA | 0/1 0% | 4/9 44.4% | 7/27 25.9% | 58/146 39.7% | 1/3 33.3 % | 2/5 40 % | 4/22 18.2 % | 7/40 17.5 % | 2/9 22.2% | 7/19 36.8% | 8/25 32% | 11/59 18.6% | 3/13 23.1% | 13/33 39.4% | 19/74 25.7% | 76/245 31% |
| AD | 0/10% | 4/9 44.4% | 11/27 40.7% | 79/146 54.1% | 1/3 33.3% | 3/5 60 % | 16/22 72.7 % | 21/40 52.5 % | 8/9 88.9% | 14/19 73.7% | 19/25 76% | 40/59 67.8% | 9/13 69.2% | 21/33 63.6% | 46/74 62.2% | 140/245 57.1% |
| CU | 1/1 100% | 5/9 55.6% | 10/27 37.0% | 105/146 71.9% | 2/3 66.7 % | 2/5 40 % | 13/22 59.1% | 29/40 72.5 % | 8/9 88.9% | 12/19 63.2% | 20/25 80% | 40/59 67.8% | 11/13 84.6% | 19/33 57.6% | 43/74 58.1% | 174/245 71% |
| FA/I | 1/1 100% | 4/9 44.4% | 10/27 37.0% | 58/146 39.7% | 1/3 33.3% | 4/5 80 % | 5/22 22.7% | 25/40 62.5% | 6/9 66.7% | 10/19 52.6% | 19/25 76% | 44/59 74.6% | 8/13 61.5% | 18/33 54.6% | 34/74 45.9% | 127/245 51.8% |
No.: number FHA: family history of allergy AR: allergic rhinitis P:pollinosis DA: drug allergy AD: atopic dermatitis CU: chronic urticaria FA: food allergy/intolerance.
Table 2 shows that severe asthma was significantly common in Group I [odds ratio (OR) (95% Confidence interval (CI)) in studied females, males and total cases: 3.10 (1.61 to 5.95), 3.02 (1.30 to 6.99), and 2.86 (1.24 to 6.59) respectively (p<0.05)].
Odds ratio and 95% CI of degree of asthma severity in Group I and Group II cases (females, males, and total)
| Females | Males | Total | |||||||
| Mild | Moderate | Severe | Mild | Moderate | Severe | Mild | Moderate | Severe | |
| Odds ratio in Group I cases (95%CI) | |||||||||
| 0.28 (0.13 – 0.61) | 0.87 (0.32–2.34) | 3.10 (1.61–5.95) | 0.24 (0.03–1.81) | 0.94 (0.27–3.28) | 3.02 (1.30–6.99) | 0.24 (0.11–0.50) | 0.94 (0.27–3.28) | 2.86 (1.24–6.59) | |
| Odds ratio in Group II cases (95%CI) | |||||||||
| 3.46 (1.62 – 7.42) | 1.14 (0.42–3.07) | 0.3225 (0.16–0.62) | 4.16 (0.55–31.40) | 1.06 (0.30 –3.69) | 0.33 (0.14–0.76) | 4.10 (1.97–8.56) | 1.06 (0.30–3.69) | 0.34 (0.15–0.80) | |
Table 3 shows that the accompanying allergic co-morbidities were significantly more common in Group I cases [allergic rhinitis (OR=2.19, 95% CI 0.89–5.37, p<0.05), pollinosis (OR=1.59, 95% CI 0.85–2.98, p<0.05), antibiotic allergy (OR=1.25, 95% CI 0.65–2.41, p<0.05), and atopic dermatitis (OR=1.34, 95% CI 0.70–2.55, p<0.05). Meanwhile, Group II showed significance prevalence of food allergy (OR=1.00, 95% CI 0.49–2.02, p<0.05) and chronic urticaria (OR=0.88, 95% CI 0.45–1.69, p<0.05). The family history of allergy status was more significant in group I cases (OR=1.27, 95% CI 0.68–2.37, p<0.05).
Odds ratio and 95% CI of allergic co-morbidities and family history of allergy in Group I and II
| Group I | Group II | |||
| Odds ratio | 95%confidence interval | Odds ratio | 95%confidence interval | |
| Food allergy | 0.99 | 0.44–2.01 | 1.00 | 0.49–2.02 |
| Allergic rhinitis | 2.19 | 0.89–5.37 | 0.45 | 0.18–1.11 |
| Pollinosis | 1.59 | 0.85–2.98 | 0.62 | 0.33–1.17 |
| Drug allergy | 1.25 | 0.65–2.41 | 0.79 | 0.41–1.52 |
| Atopic dermatitis | 1.34 | 0.70–2.55 | 0.74 | 0.39–1.42 |
| Chronic urticaria | 0.88 | 0.45–1.69 | 1.13 | 0.59–2.17 |
| Family history of allergy | 1.27 | 0.68–2.37 | 0.78 | 0.42–1.46 |
It would appear that AIA is still under-diagnosed. This might possibly be the result of the fact that patients experiencing undesirable reactions after aspirin ingestion tend to avoid taking drugs of the same kind next time round. Furthermore, in a recent European survey 15% of patients were entirely unaware of suffering from aspirin intolerance, and only provocation tests ultimately revealed their hypersensitivity. The fact that aspirin hypersensitivity frequently remains under-diagnosed might well be due to the lack of routine oral/inhaled provocation tests in everyday clinical practice.14,15
This is the first study to survey of the prevalence of AIA in Saudian asthmatics. A prevalence rate of AIA of 12.6% was reported. Studies of AIA in different populations evidenced a prevalence rate ranging from 1% to 20%, with the differences being attributed either to diagnostic methods or to differences in the populations being assessed.18–21 The study by Vally et al.5 reported the prevalence of aspirin intolerant asthma at 10–11% in three populations of asthmatics, a result very near to this study . Recently, the results of the Finnish and Australian studies, based on randomised selected population, using detailed questionnaires attested to higher prevalence of doctor-diagnosed AIA than in the Polish face-to-face interviews (8.8 and 10.9–4.3% of the adult asthmatics, respectively).5,7,18
Stevenson DD and Simon RA22 reported that 50% of the patients with AIA have chronic, severe, corticosteroid-dependent asthma; 30% have moderate asthma that can be controlled with inhaled corticosteroids; and the remaining 20% of patients have mild and intermittent asthma. However, in this study, the reported degree of severity was nearly similar in mild cases (21.7%), lower in moderate asthmatics (17.3%) and higher in those suffering from severe asthma, as evidenced in Table 2.
Although the effect of sex on the course of the disease has not been studied, the predominance of AIA among females has been observed previously.1,23–25 Similarly, the present study evidenced more prevalent disease in females (71.7%) than in males (28.3%).
Familial history of allergy was more frequent and significantly higher in Group I than Group II (OR=1.27, 95% CI 0.68–2.37, p<0.05). These data evidence that atopic background was stronger in Group I, similar to that reported by an early study,26 and controversial to another study that reported a prevalence rate of only 6% of the patients.14
In a recent epidemiological study in Finland18 the risk of aspirin intolerance causing asthma was eight times higher (only 1.16 times higher in this study) in people with allergic rhinitis than in those without it. Similarly, in Turkey,26 allergic conditions and serum IgE levels were higher in asthmatic patients with aspirin intolerance than in those who tolerated aspirin well. In the present study all studied allergic co-morbidities were significantly higher in the AIA group except for food allergy and chronic urticaria. Moreover, Samter and Beers1 and Kalyoncu et al.26 have reported more prevalent food and antibiotic allergies in AIA patients (prevalence rates of 36.3% and 22.7% for food allergy, respectively; and 7.7% and 16.7% for antibiotic allergy, respectively). In this study, antibiotic allergy reached as high as 34.8% in Group I cases, however, food allergy was significantly more prevalent in Group II cases (56.5%, OR=1.00, 95% CI 0.49–2.02, p<0.05).
A rate of 2.6% of pollinosis was reported in AIA cases in one study.7 However, in this study a high prevalence rate up to 45.7% was recorded. Meanwhile, finding an explanation to the reported low and high prevalence rates of some of the studied parameters in comparison to other studies could not be reached.
In fact, Kalyoncu et al.26 reported that some of the diseases accompanying asthma may be accepted as a risk factor for AIA, as they evidenced that other conditions related to allergy such as metal and drug allergy, chronic urticaria, dermographism, and food allergy/intolerance were all significantly more frequent in patients with AIA.
The strength of this research work is that it studied many parameters that had been studied separately in previous studies. However, one limitation of this study was the lack of provocation test as compelling evidence. Oral provocation test deserves to be performed more frequently in order to diagnose the relatively common and distinct type of asthma described here; especially as a large percentage of cases may pass unnoticed.
In conclusion, the present data demonstrate that the estimate of the prevalence of AIA appears to be closer to those reported by previous studies. This could be explained by the fact that this and other studies had combined female and male cases.1,7,16,27 Moreover, AIA in the studied population was related to asthma severity, family history of allergy and some allergic co-morbidities rather than others (such as food or with chronic urticaria). However, clearly still much has yet to be learned about both the pathogenesis and genetics of AIA. Moreover, further studies correlating questionnaire responses with the results of provocation challenge are of utmost importance to confirm the prevalence of AIA in the general asthma population.
Lastly, as evidenced by this research study, as well as by previously conducted surveys, AIA may well pose a serious health problem in need of being comprehensively addressed preferably sooner rather than later. The identification of these asthmatics is important for a number of reasons, not least of which is the fact that AIA appears to represent a distinct asthma subtype characterised by more severe and persistent asthma, an increased reliance on corticosteroid therapy, as well as an increased production of leukotrienes, and thus may have important treatment implications.14
Learning points- •
Aspirin-induced asthma is a distinct clinical syndrome affecting some asthmatic patients. It affects about 10% of adults with chronic asthma. Once developed, it runs a protracted course, despite avoidance of aspirin and cross-reacting drug, moreover, this type of asthma is not easy to treat.
- •
Unfortunately, many cases pass unnoticed, which may have catastrophic consequences for patients. As there is no documented data about the actual prevalence of this condition in the country, thus assessing its prevalence using retrospective medical records of asthmatics with determination of any characteristic association revealed close relation with other allergic co-morbidities.
- •
A larger scale study enrolling provocation test would achieve a higher rate of diagnosis.
The author declares no conflict and no financial interests.
I would like to thank all those who helped in the establishment of “Allergy Unit”, in Taif University, the first in all Saudian Universities.