Hypersensitivity reactions to aspirin and other NSAIDs occur in individuals genetically predisposed and exhibit different clinical manifestations, especially respiratory, cutaneous, and generalised. Five different phenotypes define distinct clinical pictures: aspirin-exacerbated respiratory disease, aspirin/NSAID cutaneous disease, NSAID-induced urticaria, angio-oedema and anaphylaxis, single NSAID reactions, and delayed reactions. They are observed more frequently in middle-aged women, and in atopic individuals. While ASA/NSAID hypersensitivity shares comorbidities with asthma, chronic rhinosinusitis, nasal polyposis, chronic urticaria and angio-oedema, ASA and other NSAIDs can also be cofactors for other clinically relevant conditions, especially food-dependent exercise-induced anaphylaxis, angio-oedema induced by angiotensin-converting enzyme inhibitors, and oral mite anaphylaxis. Awareness on these relationships is required for the correct diagnosis, classification, and treatment of affected patients.
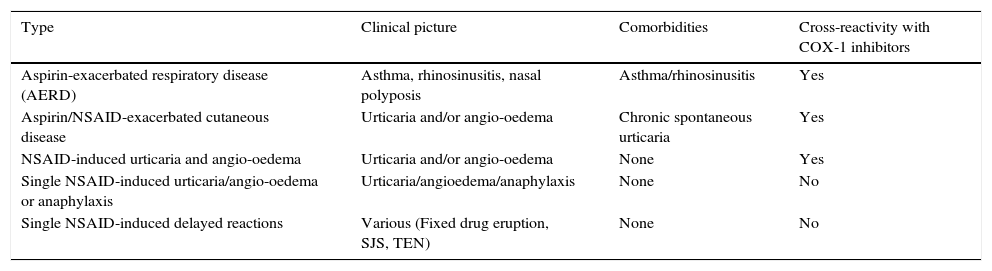
Hypersensitivity reactions to aspirin (ASA) and other non-steroidal anti-inflammatory drugs (NSAIDs) are commonly observed in clinical practice, affecting 0.3–0.5% of the population.1 Five clinical phenotypes have been described which include aspirin-exacerbated respiratory disease (AERD); aspirin/NSAID exacerbated cutaneous disease (AECD); aspirin/NSAID induced urticaria and angio-oedema; single NSAID-induced urticaria, angio-oedema and anaphylaxis; and delayed reactions (Table 1).2
Phenotypes of hypersensitivity reactions to NSAIDs.a
| Type | Clinical picture | Comorbidities | Cross-reactivity with COX-1 inhibitors |
|---|---|---|---|
| Aspirin-exacerbated respiratory disease (AERD) | Asthma, rhinosinusitis, nasal polyposis | Asthma/rhinosinusitis | Yes |
| Aspirin/NSAID-exacerbated cutaneous disease | Urticaria and/or angio-oedema | Chronic spontaneous urticaria | Yes |
| NSAID-induced urticaria and angio-oedema | Urticaria and/or angio-oedema | None | Yes |
| Single NSAID-induced urticaria/angio-oedema or anaphylaxis | Urticaria/angioedema/anaphylaxis | None | No |
| Single NSAID-induced delayed reactions | Various (Fixed drug eruption, SJS, TEN) | None | No |
SJS, Stevens–Johnson syndrome; TEN, toxic epidermal necrolysis.
Although the clinical features of NSAID hypersensitivity have been extensively investigated, little information is available in the literature about possible cofactors contributing to those reactions and the comorbidities that should be taken into account when assessing a patient complaining of adverse reactions triggered by exposure to ASA and other NSAIDs.
In this paper we will discuss various factors that are associated with NSAID hypersensitivity, its relationship with atopy, patient's gender and age. Comorbidities and the association with food-dependent exercise-induced anaphylaxis and oral mite anaphylaxis will also be reviewed.
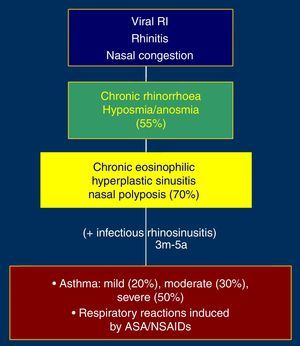
Cofactors and comorbidities in aspirin-exacerbated respiratory disease (AERD)AERD affects 1–2% of the general population, 5–7% of asthmatics, is present in 2–25% of patients with severe asthma, and in 26–40% of patients who have the triad asthma-chronic rhinosinusitis-nasal polyposis. Also, about 15% of patients with nasal polyps are sensitive to aspirin. This condition usually begins as a flu-like syndrome between adolescence and 40 years of age, being more prevalent in females. Positive immediate hypersensitivity skin tests to inhalant allergens are present in one third to two thirds of AERD patients.
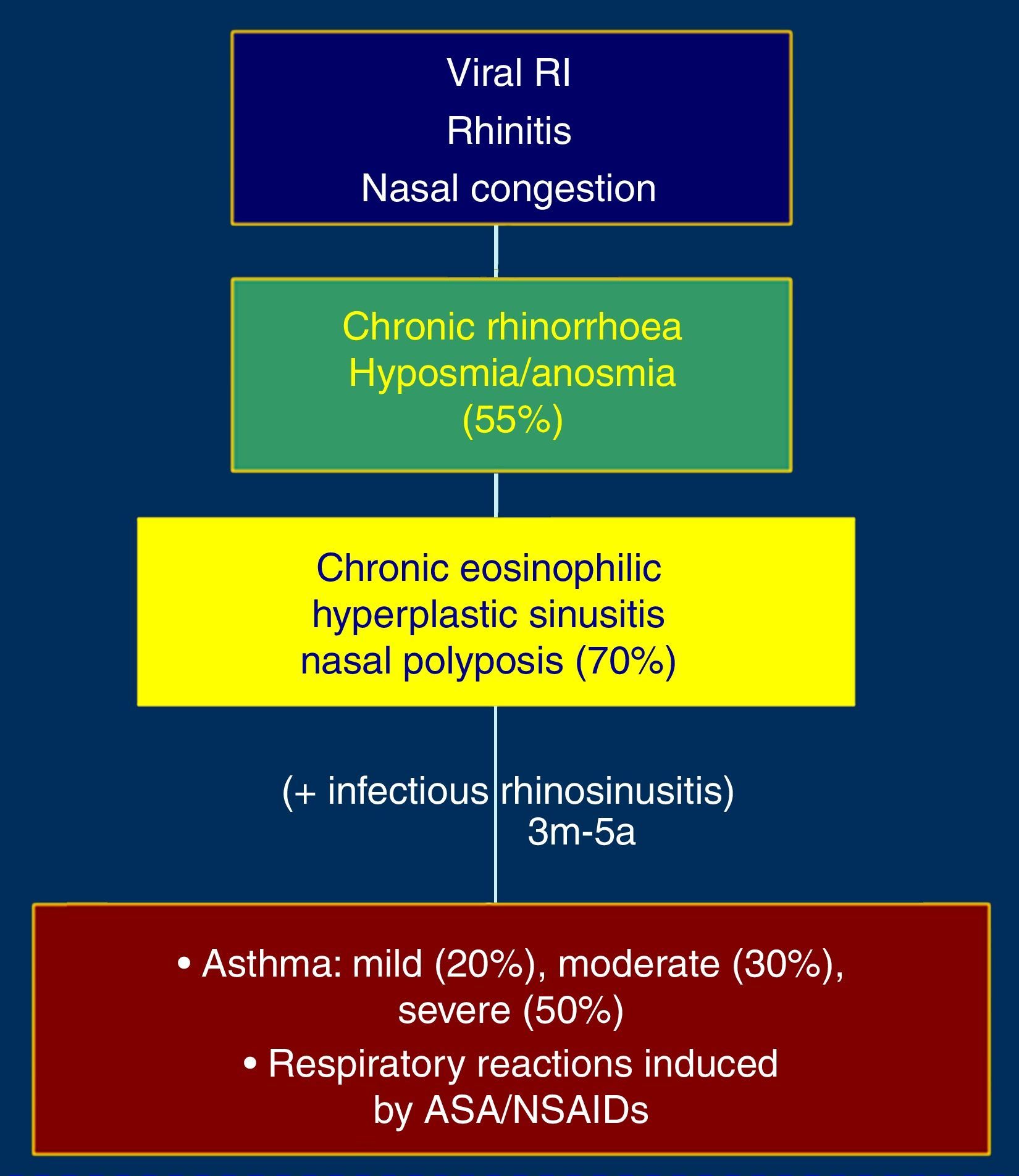
The initial picture is characterised by rhinitis and nasal congestion which is followed by chronic eosinophilic and hyperplastic pansinusitis, hyposmia, nasal polyposis and asthma (Fig. 1). Severity of asthma in these patients is moderate in 30% and severe in 50% of the cases. Exposure to aspirin or NSAIDs that inhibit cyclooxygenase-1 (COX-1) induces asthmatic exacerbations which can be life-threatening. Asthma observed in patients with AERD is frequently steroid-dependent.3,4
The mechanisms responsible for acute respiratory reactions to ASA/NSAIDs involve the inhibition of COX-1 isoenzyme, resulting in a decreased production of PGE2 and increased levels of cysteinyl-leukotrienes. On the other hand, the chronic eosinophilic inflammation of the upper and lower respiratory tract is mediated by an increased synthesis of cytokines with effects on eosinophils (IL-5, GM-CSF, RANTES, eotaxin). An increase of γ-interferon production by nasal polyp tissues has also been demonstrated.
The diagnosis of AERD is based on the medical history, nasal endoscopy, CT scan of paranasal sinuses, and aspirin challenge tests. For provocation tests the oral route constitutes the gold standard, although protocols for nasal and bronchial provocation tests are also utilised in many centres. The management of patients with AERD is summarised in Table 2.
Management of aspirin-exacerbated respiratory disease.
| • Avoidance of COX-1 inhibitors |
| • Use of alternative analgesic and anti-inflammatory drugs: acetaminophen, nimesulide, meloxicam, coxibs |
| • Opioids for severe and post-operative pain |
| • Corticosteroids: inhaled, intranasal, oral |
| • Leukotriene modifiers: leukotriene receptor antagonists, 5-LO inhibitors |
| • Treatment for chronic rhinosinusitis: antibiotics, antifungals |
| • Treatment of allergic disease, if present |
| • Surgery |
| • Desensitisation |
| • Under investigation: Omalizumab, Ligelizumab, Reslizumab, Mepolizumab, GATA-3, DNAZyme, genetically-modified Lactococcus lactis, Prasugrel, Ifetroban |
ASA and NSAIDs can induce acute urticaria.2 Additionally, an important proportion of up to 40% of patients affected by chronic spontaneous urticaria experience exacerbations of the underlying disease when exposed to ASA or classic NSAIDs.5 These cutaneous reactions generally occur between 0.5 and 6h after the drug intake, and the mechanism is similar to that of acute respiratory reactions, involving COX-1 inhibition and increased production of leukotrienes. The diagnosis is made through the medical history, physical examination, and oral provocation tests. Management of NSAID-induced urticaria and angio-oedema is presented in Table 3.
Management of urticaria and angio-oedema induced by ASA/NSAIDs and aspirin-exacerbated cutaneous disease.
| • Avoidance of COX-1 inhibitors |
| • Alternative analgesic and anti-inflammatory drugs: Acetaminofen, nimesulide, meloxicam, coxibs |
| • Opioids for severe and post-operative pain |
| • Treatment of chronic urticaria according to current guidelines6 |
| • Desensitisation not usually recommended |
Demographic features. According to various studies from our group between 67.0 and 79.3% of patients who suffer urticaria and AE induced by NSAIDs are females with a mean age ranging from 28.6 to 32.7 years.7–9
Atopy. Hoigne and Szczeklik proposed in 1992 that atopy predisposes to more severe reactions to NSAIDs.10 In 1996 Quiralte et al. described the association of NSAID hypersensitivity and periorbital angio-oedema.11 Sánchez-Borges and Capriles observed that the prevalence of atopy is increased in NSAID-sensitive individuals and represents a risk factor for NSAID hypersensitivity.7 This observation was confirmed by Rosario and Ribeiro.12
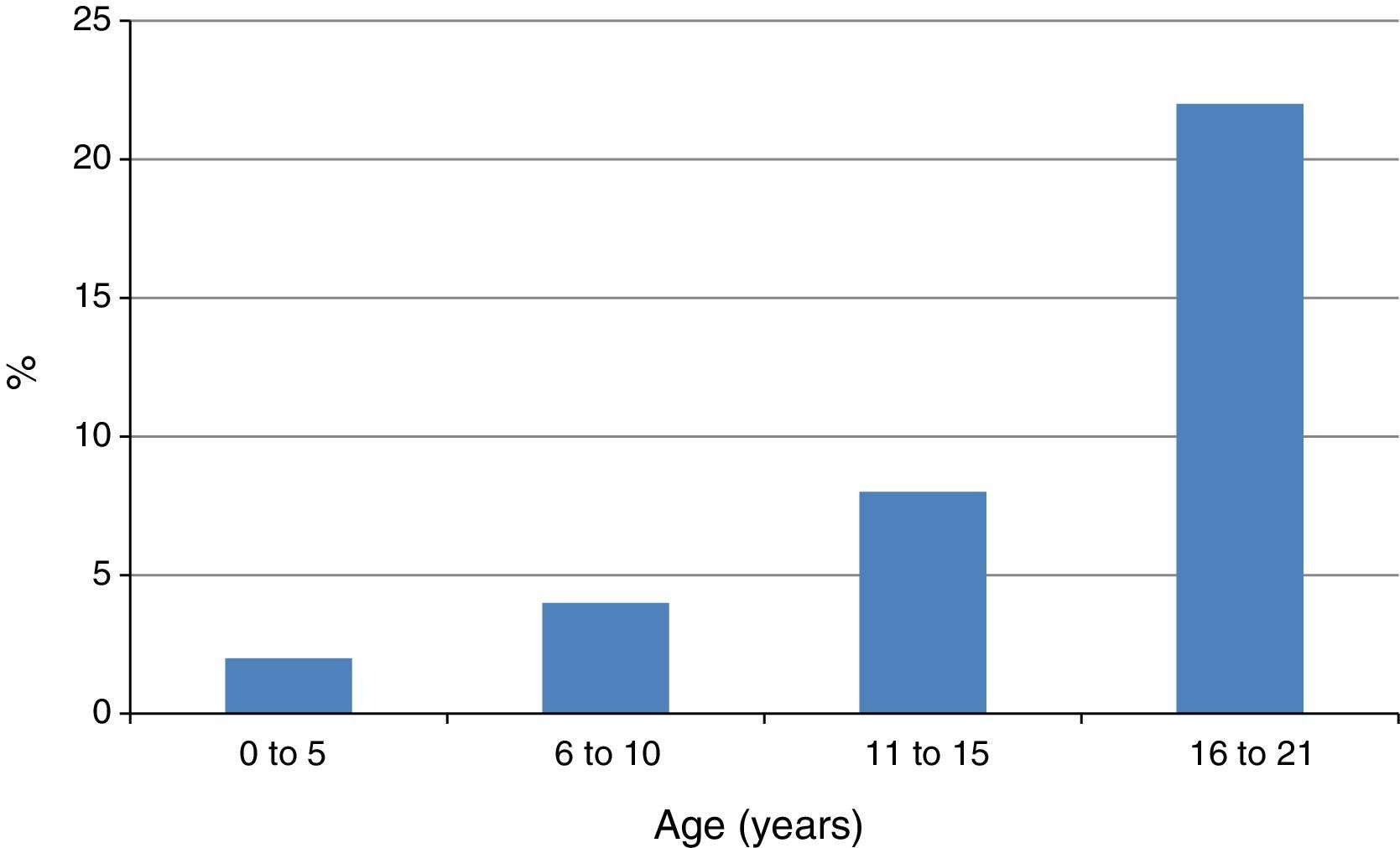
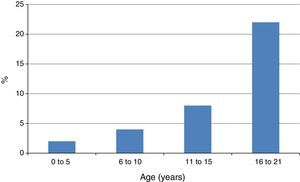
A related finding by Capriles et al. was the progressive increase of the frequency of NSAID-induced AE in atopic children from early childhood until adolescence13 (Fig. 2). In our studies the rate of atopy among patients with NSAID-triggered urticaria and AE has been consistently very high, between 83.3 and 98.0%.7–9
Another related observation is that patients with U/AE induced by NSAIDs have increased levels of total serum IgE, as well as specific IgE to Dermatophagoides pteronyssinus and Blomia tropicalis, when compared with healthy controls.14
Interestingly, patients with aspirin-exacerbated cutaneous disease (a subtype of chronic spontaneous urticaria in which acute exacerbations of the underlying disease are observed when the patient receives COX-1 inhibitors) show an increased frequency of angio-oedema, positive immediate type skin tests to inhalant allergens, and longer disease duration as compared with NSAID-tolerant patients with chronic urticaria (CU).5 In agreement with these results, a Korean study found that atopy was significantly more prevalent in aspirin-intolerant than in aspirin-tolerant CU patients.15 In fact, current guidelines for the management of CU recommend stopping NSAIDs in patients taking those medications.6
Risk factors for anaphylaxis induced by NSAIDsAnaphylactic reactions due to ASA and NSAIDs are associated with some particular risk factors. Among them, the following have been frequently mentioned:16 (1) Use of glafenin, pyrazolones, and heteroaryl acetic acids (diclofenac, ketorolac, tolmetin). (2) Female gender. (3) Use of NSAIDs in patients with acute pain. (4) Atopy. (5) Mastocytosis.
NSAIDs and food allergyNSAID consumption constitutes a significant risk factor for the development of severe IgE-dependent food allergy, and especially food-dependent exercise-induced anaphylaxis (FDEIA).17 This association has led authors to propose that adult patients allergic to foods should avoid aspirin and NSAIDs before meals.18,19
Most frequently foods inducing FDEIA are wheat, vegetables, shellfish, fruits, nuts, egg, mushrooms, corn, garlic, pork, beef, rice, and cow's milk. In Europe the foods most commonly involved in FDEIA are vegetables containing lipid transfer proteins (LTPs),20–23 whereas in Japan, wheat allergens, especially ω-5 gliadin and glutenin, are involved in most cases.
NSAIDs are important cofactors in the induction of approximately 40–58% of FDEIA associated to LTPs. It may also require the concomitant participation of other cofactors such as physical exercise, and alcohol.24,25 Aspirin induces symptoms in combination with food ingestion even without exercise challenge.26,27 In addition, it has been reported that in patients with food-dependent exercise-induced anaphylaxis (FDEIA) in which food provocation tests are negative, aspirin ingestion (with or without exercise) may result in a positive test.28–30
The most likely mechanism for NSAID-associated FDEIA is the increase of gastrointestinal permeability, which can be induced by ASA as well as by exercise and alcohol, and can facilitate allergen absorption.31 In fact, aspirin increases serum levels of circulating gliadin peptides, and blood gliadin levels correlate with symptoms.32 NSAIDs also enhance in vitro histamine release induced by IgE-mediated mechanisms,33 and it has been observed that aspirin stimulates histamine release from mast cells and basophils through the suppression of prostaglandin E2 production, which regulates the release of histamine.34 Other cofactors associated to FDEIA different to aspirin, alcohol, and exercise have been described, including fatigue, lack of sleep, common cold, stress, menstruation, and atmospheric conditions.
In a recent paper the transcriptomic analysis has suggested the presence of disturbances of intestinal homeostasis in FDEIA. The authors suggested that FcγRI and LTP-specific IgG might contribute to anaphylaxis induced by LTPs, whereas adenosin receptor 3 (ADORA3) could be related to reactions involving NSAIDs.35
NSAIDs as cofactors of ACEi-induced angio-oedemaMore than 40 million patients worldwide are currently treated with Angiotensin Converting Enzyme inhibitors (ACEIs), and in 2011 there were 164 million prescriptions of ACEIs in the USA. The prevalence of angio-oedema in patients receiving ACEis is 0.1–6%.36 In some studies, ACEis were the most common cause of AE (24–58% of all cases of AE),37–39 being more prevalent in black individuals, and life-threatening in 40% of the cases with a mortality rate of 0.1%.
The frequent concomitant use of aspirin and other NSAIDs in patients receiving ACEIs seems to enhance the risk of angio-oedema.40,41 In a small series of patients with ACEI-induced angio-oedema we observed that five out of nine patients were receiving NSAIDs concurrently with ACEIs.42 The recommendation has been made to carefully observe patients receiving simultaneously ACEIs and NSAIDs in order to establish as early as possible any signs of AE in this patient population.
Association of NSAID hypersensitivity with oral mite anaphylaxisOral mite anaphylaxis (pancake syndrome) is a clinical picture of severe systemic reactions induced by the ingestion of mite-contaminated foods, generally prepared with wheat flour, and more often pancakes. Currently there are reported in the literature 170 cases of this clinical condition, involving patients of any age or gender from Europe, North America, Central and South America and Asia. Seventy-five out of those 170 patients (44.1%) also had NSAID hypersensitivity and especially NSAID-induced angio-oedema.43–45
The reasons for this comorbidity are not presently known, although various possible mechanisms have been proposed, including IgE-mediated reactions, stimulation of innate immunity, inhibition of cyclooxygenase-1 by mite-derived components, direct stimulation of mast cell receptors, Dectin-glycan interactions, and tissue damage with increased allergen absorption induced by mite peptidases.46
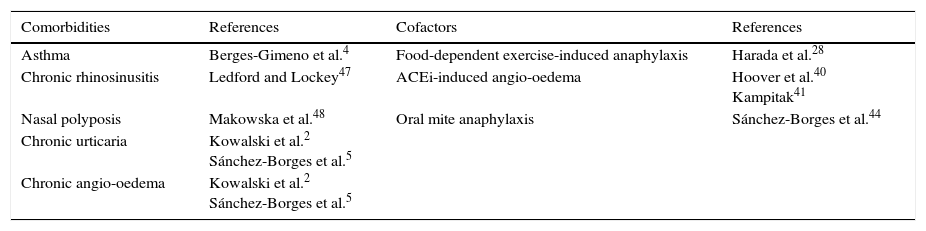
ConclusionsHypersensitivity reactions to aspirin and other COX-1 inhibitors occur in individuals genetically predisposed and exhibit different clinical manifestations, especially respiratory, cutaneous, and generalised. They are more frequent in middle-aged women, and atopic individuals. While ASA/NSAID hypersensitivity shares comorbidities with asthma, chronic rhinosinusitis, nasal polyposis, chronic urticaria and angio-oedema, ASA and other NSAIDs can also be cofactors for other clinically relevant conditions. Those include food-dependent exercise-induced anaphylaxis, angio-oedema induced by angiotensin-converting enzyme inhibitors, and oral mite anaphylaxis. Clinicians should investigate these associations and comorbidities in order to provide optimal management to patients suffering the above mentioned diseases (Table 4).
Comorbidities and cofactors of aspirin/NSAID hypersensitivity.
| Comorbidities | References | Cofactors | References |
|---|---|---|---|
| Asthma | Berges-Gimeno et al.4 | Food-dependent exercise-induced anaphylaxis | Harada et al.28 |
| Chronic rhinosinusitis | Ledford and Lockey47 | ACEi-induced angio-oedema | Hoover et al.40 Kampitak41 |
| Nasal polyposis | Makowska et al.48 | Oral mite anaphylaxis | Sánchez-Borges et al.44 |
| Chronic urticaria | Kowalski et al.2 Sánchez-Borges et al.5 | ||
| Chronic angio-oedema | Kowalski et al.2 Sánchez-Borges et al.5 |
The authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Protection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals for this investigation.
Conflict of interestThe authors have no conflict of interest to declare.