We aimed to observe the effect of icariin on an asthma mouse model and explore the potential underlying mechanisms.
MethodsThe asthma mouse model was established by ovalbumin (OVA) sensitisation and respiratory syncytial virus (RSV) infection and then treated with icariin. Airway resistance was assessed by whole body plethysmograph. In addition, pathological slides were stained with haematoxylin–eosin, and the peribronchial inflammation was observed microscopically. The concentration of prostaglandin D2 (PGD2) in serum and bronchoalveolar lavage fluid (BALF) was detected by enzyme-linked immuno sorbent assay (ELISA). The relative level of prostaglandin D2 receptor 2 (CRTH2) mRNA was assessed by real-time quantitative PCR.
ResultsCompared with the icariin-untreated group, there was a significant reduction of Penh in the treated group. Total leucocyte amount and all sorts of leukocytes were lower in the treated group than in the untreated group. HE staining results revealed that a large number of inflammatory cells infiltrated into the peribronchial tissues of untreated group, and the degree of airway inflammation decreased significantly in the treated group. PGD2 in serum and BALF, as well as CRTH2 mRNA level in lung tissues were lower in the treated group than in the untreated group.
ConclusionIcariin is a promising therapeutic strategy for asthma, and PGD2 might be a new target for asthma therapy in OVA-induced and RSV-infected asthma model.
Bronchial asthma is a chronic inflammatory disease of the airway, involving many cellular elements and cells, such as eosinophils, mast cells, T lymphocytes, neutrophils and airway epithelial cells.1 The prevalence and mortality of asthma is in an upward trend, which makes this chronic disease one of the main serious problems to public health.2 The main treatments for asthma are non-specific anti-inflammatory drugs and bronchodilators.3 In recent years, developing therapy methods are emerging, including anti-IgE therapy, leukotriene modifying drugs, immunotherapy and bronchial thermoplasty.4 However, these methods should be improved with respect to cost and/or side effects.
Icariin is one of the main ingredients of Herba epimedii, with the ability of relieving asthma and cough, anti-allergy activity and enhancing humoral immunity and cellular immunity.5 Modern pharmacological studies indicate that icariin was also associated with immune regulation, anti-bacterial and anti-asthmatic effects.6 It could significantly boost immunity of the respiratory tract, improve asthmatic symptoms, reduce the production of inflammatory mediators, improve the endogenous anti-inflammatory ability of the body, shorten the sustaining duration, and reduce the frequency of asthma. Moreover, icariin is confirmed to be effective in the therapy of respiratory syncytial virus (RSV)-induced asthma.7,8 However, the specific mechanism remains unclear.
In order to verify the therapeutic effect of icariin on asthma and to explore the underlying mechanisms, a mouse asthma model was constructed and treated with icariin in this study. The airway resistance and the pathological feature of lung tissue were observed. Simultaneously, the concentration of prostaglandin D2 (PGD2) and relative level of prostaglandin D2 receptor 2 (CRTH2) mRNA were assessed.
Materials and methodsAnimalsA total of 30 four-week-old female specific-pathogen-free BALB/c mice, weighing 16–19g, were purchased from Shanghai SLAC Laboratory Animal Co., Ltd. These animals received food and water ad libitum in a reversed 12:12-h light/dark cycle. Two weeks later, these mice were randomly divided into three groups, ovalbumin (OVA)/RSV-Y group, OVA/RSV-N group, and the control group (n=10 in each group). The asthma model was constructed in mice of the OVA/RSV-Y group and OVA/RSV-N group. Normal mice in the control group served as blank control. All animal care and experimentation were performed in compliance with the Regulations for Administration of Affairs Concerning Experimental Animals (China, 2010), and approved by the Animal Ethics Committee of Shanghai Jiao Tong University.
Model construction and interventionThe asthma model was constructed in both the OVA/RSV-Y group and the OVA/RSV-N group with OVA sensitisation. In detail, mice were firstly anaesthetised by methoxyflurane inhalation, and then immunised on days 0 and 7 by intraperitoneal injection with 20μg OVA (GradeV; Sigma–Aldrich, St. Louis, USA) emulsified in 200μl of PBS buffer containing 2.25mg aluminium hydroxide adjuvant. From days 14 to 16, 25μl RSV (Shanghai Genechem Co., Ltd., China) with a titre of 107pfu was dropped nasally to each mouse. RSV titre in lung tissues were examined on day 17. To verify whether the asthma model was successfully constructed, three mice from each of these two groups were sacrificed and pulmonary function was tested. From days 22 to 24 and days 29 to 31, each mouse was challenged by nasally dropping 25μl RSV at the titre of 107pfu. Then, 2.5mg icariin (Kailai Biotech Co., Ltd., China) in 200μl normal saline was intraperitoneally injected to mice in the OVA/RSV-Y group once a week for two consecutive weeks. An equal volume of sterile water was administered to mice in the control group through the whole procedure.
RSV titres in lung tissueRSV titres were detected on day 17 as follows. Lungs of three mice in both the OVA/RSV-Y group and the OVA/RSV-N group were aseptically removed. Approximately 0.1g lung tissue was homogenised in 1ml Dulbecco's PBS (GIBCO), and then added to confluent monolayers of Vero cells for RSV titre detection. Thereafter, the monolayer was cultured at 37°C for three days, and RSV plaques were calculated after immunostaining with monoclonal antibodies against the G and F glycoproteins as described in previous studies.9,10
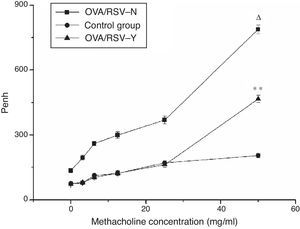
Airway reactivity measurementOn days 21 and 35, airway responsiveness to methacholine (Mch, Sigma–Aldrich, St. Louis, USA) was assessed by whole-body plethysmography (WBP, Buxco Electronics Inc., Troy, NY, USA) in a dose-dependent manner (3.125, 6.25, 12.5, 25, 50mg/ml of methacholine in PBS). Airway reactivity, as the indicator for respiratory function, was determined by interpolation of the dose–response curve for enhanced pause (Penh).
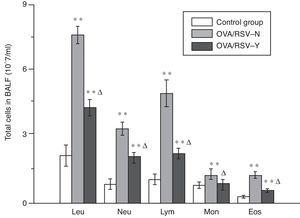
Cell identification and PGD2 measurement of bronchoalveolar lavage fluidAfter airway reactivity measurement, the mice's trachea was cannulated by 22G venous indwelling needle in a supine position. The right lung was ligated at the right main branch of the trachea using surgical forceps, and the left lung was lavaged by tracheal cannula with 0.5ml phosphate-buffered saline (PBS, Sigma–Aldrich, St. Louis, USA) for three times. The retrieved bronchoalveolar lavage fluid (BLAF) was pooled and centrifuged at 2000r/min for 10min at 4°C. The supernatant was collected and stored at −80°C for PGD2 level detection and cell identification. Moreover, blood was collected by enucleation of eyeball, and serum was separated in a refrigerated centrifuge at 4°C and stored at −80°C. In this study, PGD2 level in serum and BLAF was detected using the Enzyme-linked Immunosorbent Assay (ELISA) kit (Uscn Life Science Inc., China), and the leucocyte were counted and classified into neutrophil (Neu), lymphocyte (Lym), monocyte (Mon), eosinophil (Eos) and basophil (Bas) by BC-2800 Vet blood analyser (Mindray, China).
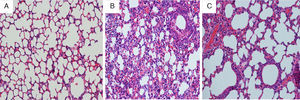
Immunohistochemical analysis for lung tissueThe right lobe of the lung was inflated and fixed immediately in 10% neutral buffered formalin for 24h, followed by dehydration, transparency and paraffin embedding. The blocks were cut into 5μm segments. Airway inflammation was assessed by haematoxylin and eosin staining. Histological sections were blindly observed using a light microscope.
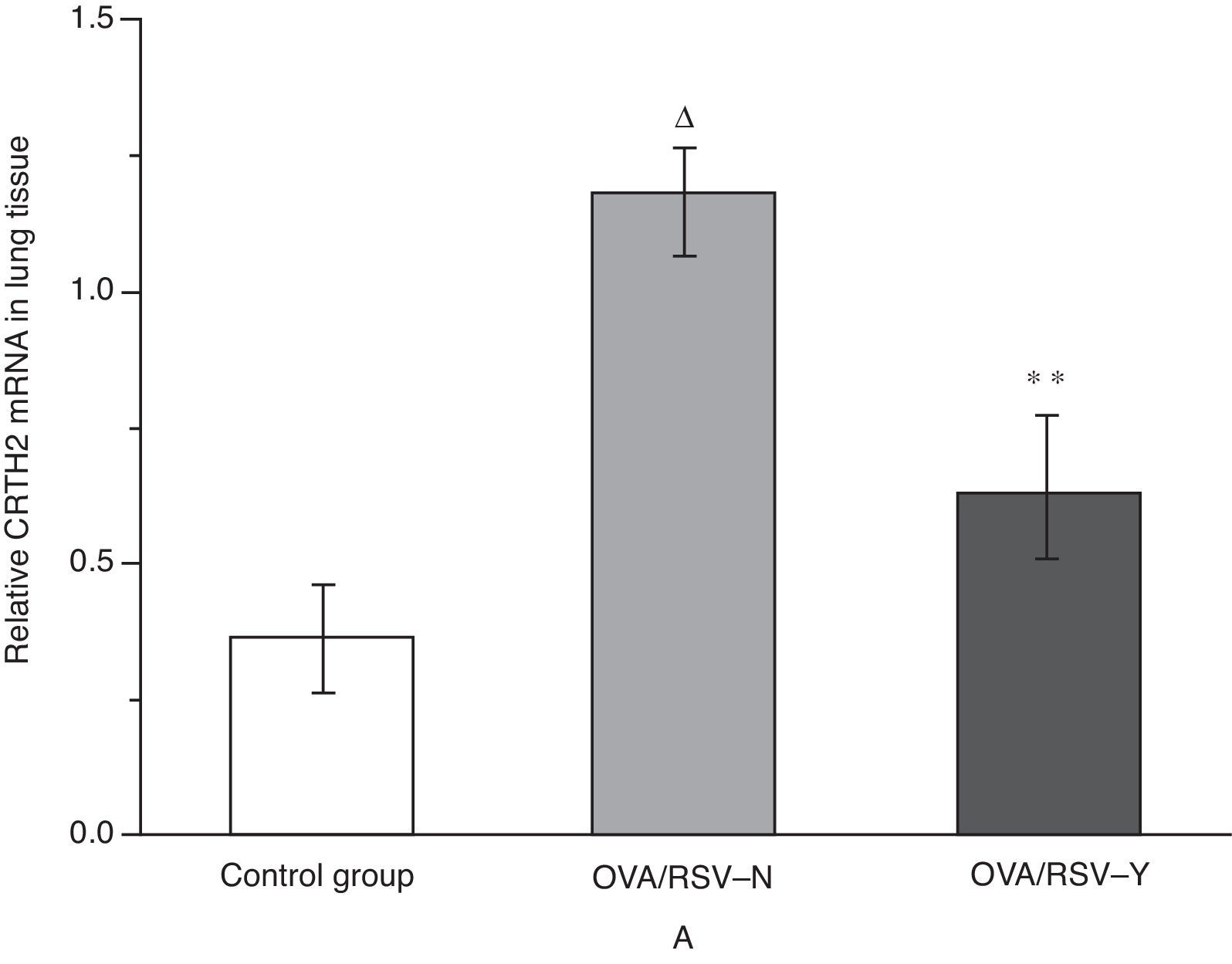
Real-time quantitative PCR for CRTH2 levelBased on the manufacturer's instructions, total RNA was extracted and purified from lung tissue with Trizol reagent (Invitrogen). The concentration and purity were measured by the spectrophotometric absorbance at 260 and 280nm. Reverse transcription process of RNA to cDNA was undertaken by adding 2μg RNA to a solution with an oligo-dT primer and reverse transcriptase (Promega Co., USA). Quantitative real-time PCR was performed in a total volume of 20μl containing 1μl template DNA, 1μl sense and antisense primers, 7μl H2O and 10μl SYBR Green Master Mix (ABI, USA) using the STRATAGENE MX3000 system (Agilent, USA). The primers were designed as follows: β-actin: sense 5′-CTG TCCCTG TAT GCC TCTG-3′, antisense 5′-TGT CACGCACGATTT CC-3′; CRTH2: sense 5′-TAT CCG ACT TGT TAG CCG CC-3′, antisense 5′-TTG CAG AAGGTAGTG CCC AG-3′. After incubation at 95°C for 10min, the mixtures were subjected to 40 cycles of 30s at 95°C, 30s at 57°C and 45s at 75°C. The fold change of each gene was calculated using the ΔΔCt method with β-actin gene as an internal control. The final data were presented as fold change compared to the gene expression of mice in the normal control group.
Statistical analysisStatistical analysis was performed using SPSS 19.0 software (version 19.0; SPSS Inc., Chicago, IL, USA). The experimental data were represented as mean±standard deviation (SD). Student's t-test was used for the intergroup comparison. P<0.05 was considered to be statistically significant.
ResultsGeneral observationAfter being sensitised by OVA and infected by RSV, the mice in the OVA/RSV-Y group and the OVA/RSV-N group showed asthma symptoms including piloerection, dysphoria, scratching head and back, polypnea, nodding breathing and abdominal muscle twitch compared with those in the control group. After icariin treatment, symptoms of dysphoria and polypnea were alleviated in the OVA/RSV-Y group compared with those in the OVA/RSV-N group.
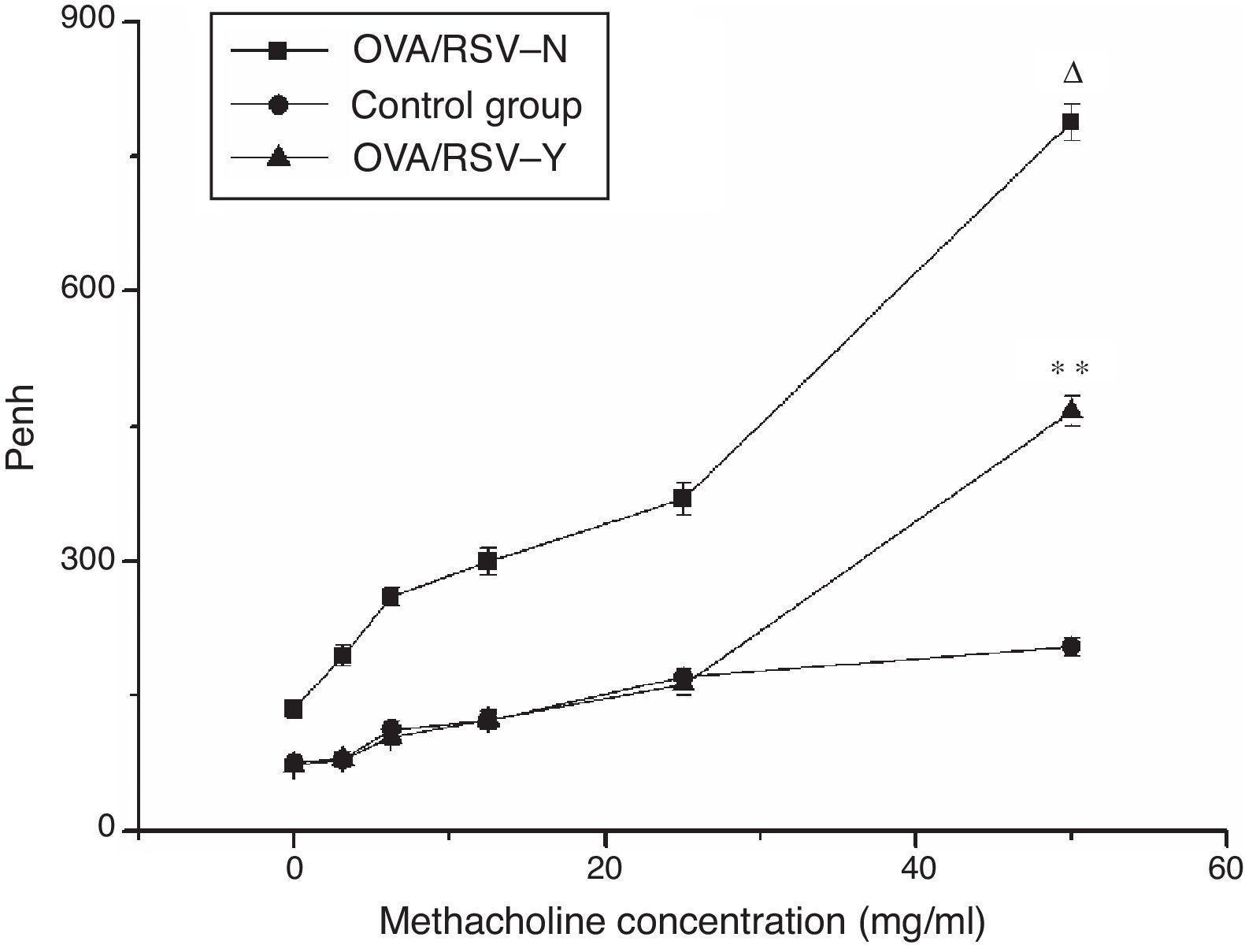
Lung functional testIn the three groups, the Penh of mice was increased with the methacholine concentration. Compared with the control group, Penh of mice in the OVA/RSV-Y and OVA/RSV-N groups was significantly higher (P<0.05). In addition, the Penh in the OVA/RSV-Y group was significantly higher than that in the OVA/RSV-N group (Fig. 1). These results indicated that icariin could decrease the Penh in mice asthma model.
The relationship between Penh and the methacholine concentration in the OVA/RSV-Y, OVA/RSV-N, and control groups. Note: ** represents P<0.01 when the OVA/RSV-Y group was compared with the OVA/RSV-N group; Δ represents P<0.01 when the OVA/RSV-N group was compared with the control group.
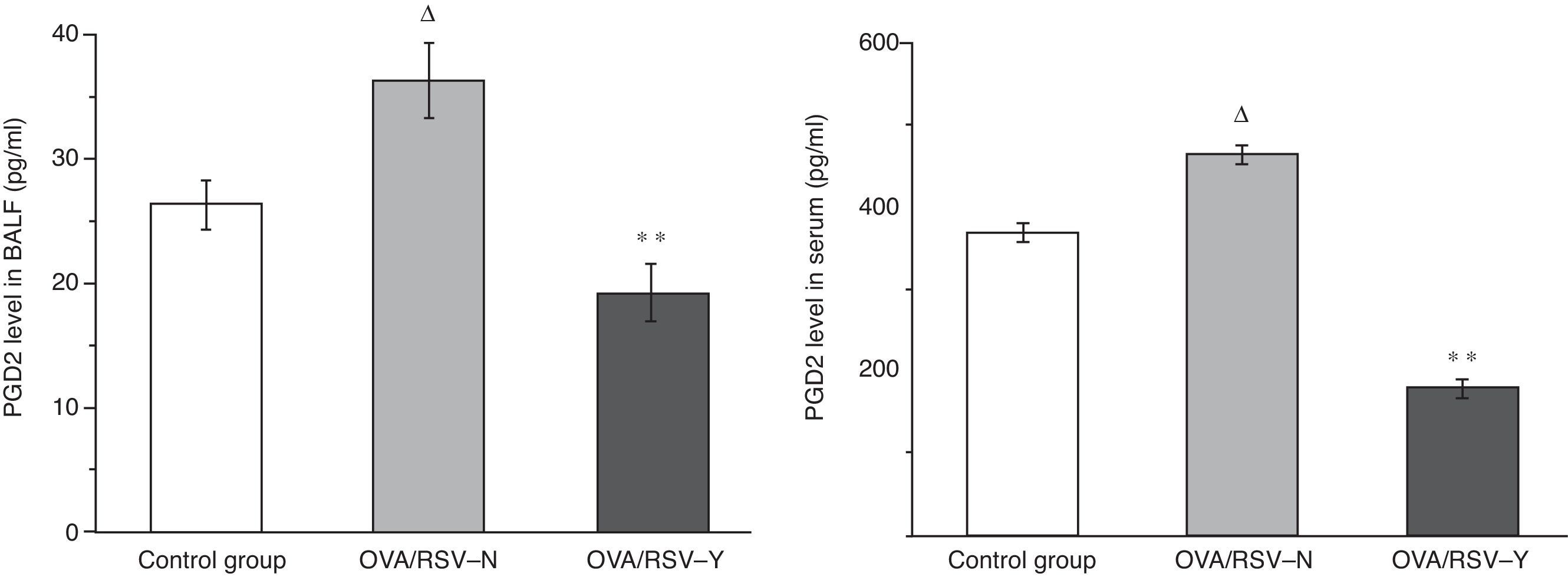
As shown in Fig. 2, in both serum and BALF, the PGD2 level was higher in the OVA/RSV-N group than that in control group (P<0.01), while the PGD2 level in the OVA/RSV-Y group was significantly lower than that in the OVA/RSV-N group (P<0.01). The results showed that the PGD2 level increased in the mouse asthma model, and decreased by icariin treatment.
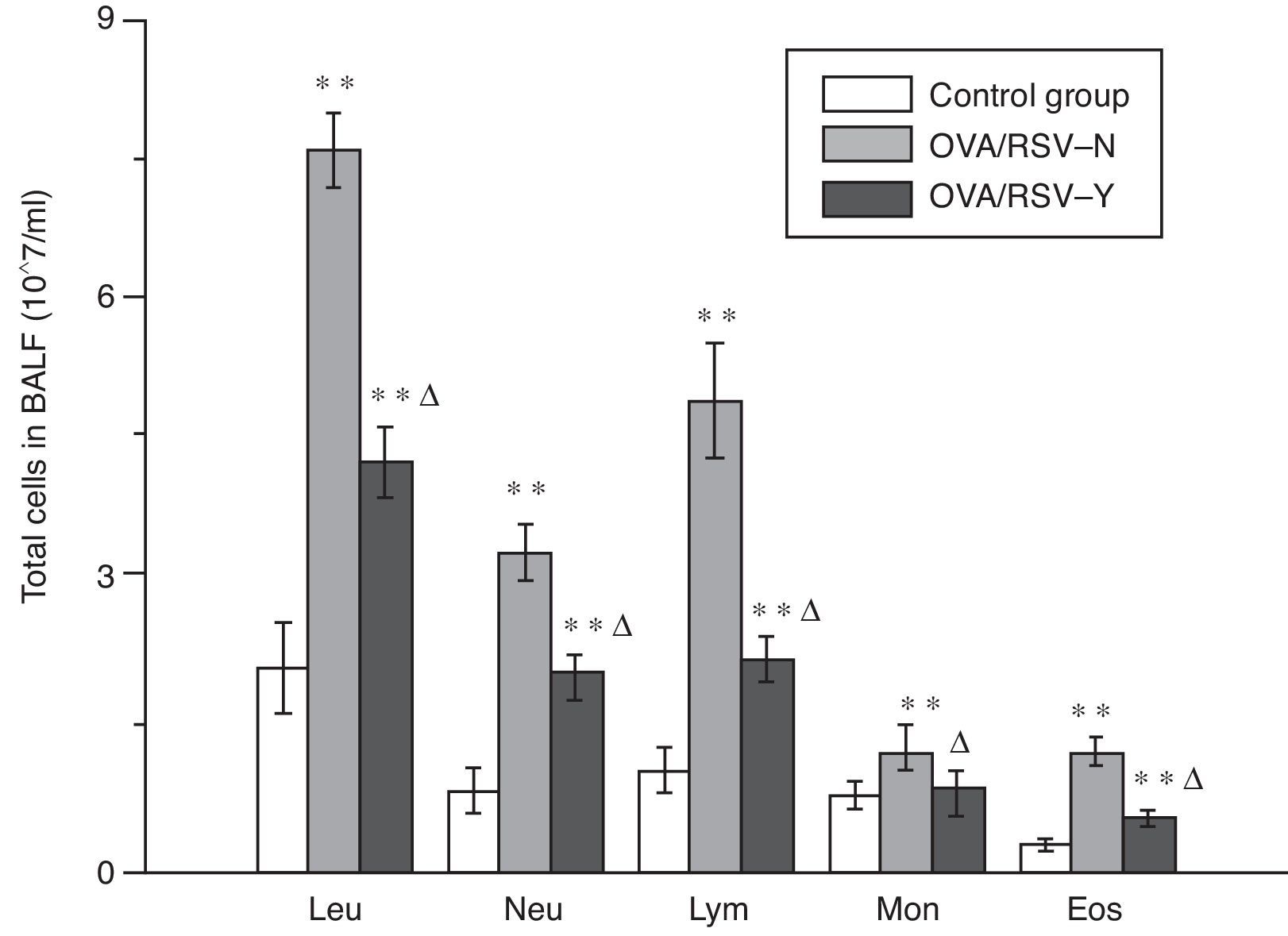
Cell classification and count in BALFAs shown in Fig. 3, the amount of each type of leucocytes significantly increased in BALF of mice in the OVA/RSV-Y and OVA/RSV-N group, while icariin could significantly decrease the leucocyte amount. However, the amount was still significantly higher in the OVA/RSV-Y group than that in the control group.
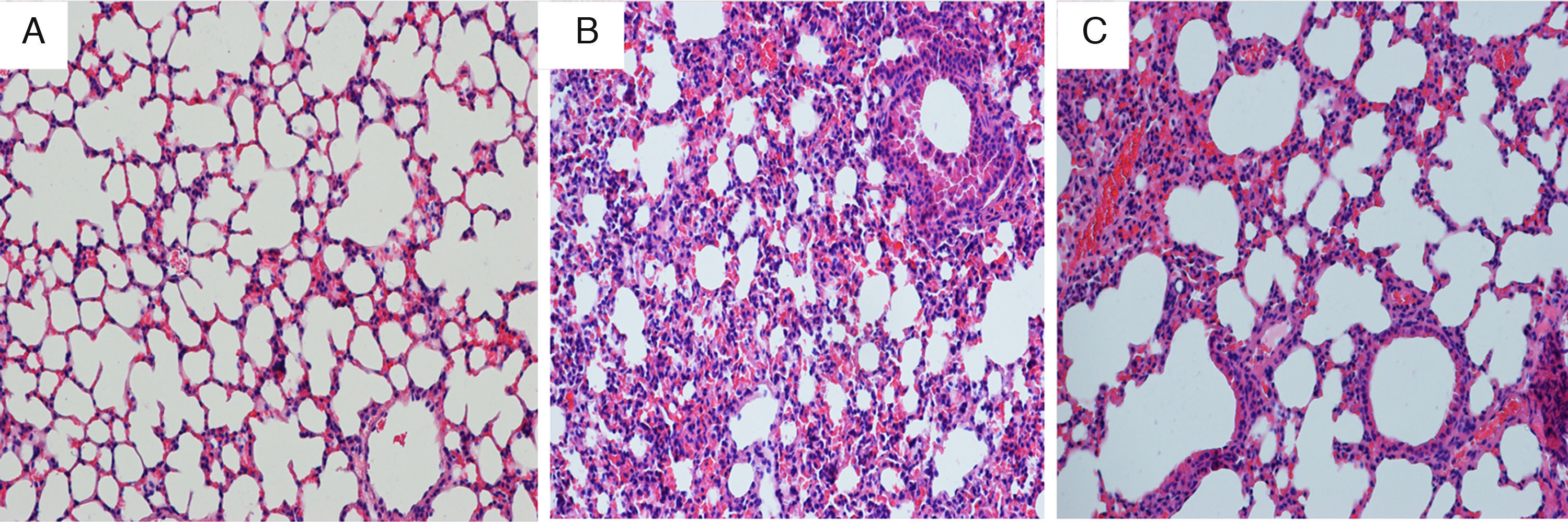
Immunohistochemical analysis for lung tissueCompared with mice in the control group, the tracheal wall in the OVA/RSV-N group was obviously thickened, and the lumen of the airway was narrowed. In addition, massive inflammatory cells including neutrophils, lymphocytes and monocyte infiltrated the airway. However, few eosinophils were observed. After treatment with icariin, there was a remission in the thickened walls and narrowed lumens of the airway, and inflammatory cellular infiltration was alleviated (Fig. 4).
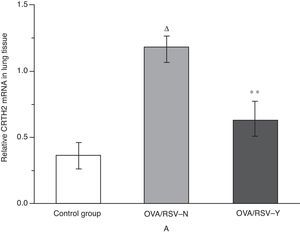
Effects of icariin on CRTH2 mRNA level in lung tissueAs shown in Fig. 5, real-time PCR revealed significantly higher level of CRTH2 mRNA in the OVA/RSV group compared with that in the control group (P<0.01). Besides, icariin treatment significantly reduced the CRTH2 mRNA level in the OVA/RSV-Y group when compared with that in the OVA/RSV-N group.
DiscussionIn this study, an asthma model sensitised by OVA and infected by RSV was successfully established. After treatment with icariin, lung function of asthmatic mice was significantly improved, and the thickened walls and narrow lumens of the airway, as well as the infiltration of inflammatory cells in lung tissue were relieved to some extent. Meanwhile, all sorts of leukocytes in BALF, PGD2 level in both serum and BALF, and the CRTH2 mRNA level in lung tissue were obviously decreased following icariin treatment.
A previous study has confirmed that OVA could induce a significant increase in airway resistance and also weaken the lung dynamic compliance.11 Respiratory viral infection plays an important role in the acute attack and exacerbation of bronchial asthma.12 For people with susceptibility factors for asthma, exposure to RSV may directly contribute to asthma or at least invoke pathophysiological changes in lung tissues.13 In turn, activation of immune responses during RSV infection can increase the susceptibility to asthma.14,15 Additionally, there was a significant leucocyte influx into BALF in the OVA-induced asthma model.16 Previous studies confirmed that icariin could also reduce inflammatory cytokine infiltration and recover the ability of glucocorticoid responsiveness.17 Moreover, icariin has been confirmed to possess the anti-inflammatory activities and weaken the inflammation in an asthma model.18,19 In this present study, icariin was found to prominently improve asthmatic symptoms and lung function, which was in line with the previous studies.
In the complex mechanisms of asthma, various factors have been revealed to lay a foundation for asthma treatment. For instance, a previous study demonstrated that the effect of icariin on the attenuation of airway hyperresponsiveness and chronic airway inflammatory changes in OVA-induced murine asthma model may be associated with regulation of Th17/Treg responses.20 PGD2, mostly derived from mast-cell activation, is an important inflammatory mediator. It could not only evoke intense contraction of bronchia, but also increase the capillary permeability.21 PGD2 is considered to be responsible for bronchoconstriction, eosinophils infiltration and mucosa secretion in allergic asthma. Moreover, increased PGD2 could also promote chemotaxis of eosinophils and makes eosinophils infiltrate into the airway resulting in eosinophils infiltration in the pathology of asthma.22 In addition, it regulated fibroblast formation and transformation, and also participated in the airway remodelling in the same time.23 The changes of PGD2 lead to the accumulation of cytokines and chemotaxin, lymphocytes and eosinophils, and further induce a series of anaphylaxis. Besides, the level of PGD2 and its metabolites in BALF of asthmatic patients has been found to be increased, especially after challenged by allergens such as CRTH2.24–27 CRTH2 is one of PGD2 receptors, and PGD2 could promote eosinophils responding and matrix contraction via acting with CRTH2, and further regulate tissue repair.28,29 Endogenous PGD2 produced by various causes may trigger an inflammatory reaction through the enhanced expression of CRTH2 in eosinophils.30 In the present study, PGD2 in serum and BALF and the CRTH2 mRNA level in lung tissue were found to be significantly increased in asthmatic mice. After treatment with icariin, the relative levels of PGD2 and CRTH2 were all decreased, which indicates that the therapeutic effects of icariin on asthma may be associated with the regulation of PGD2.
In conclusion, icariin treatment may be a promising therapeutic strategy for asthma, and PGD2 may be a new target for asthma therapy in OVA-induced and RSV-infected asthma model.
Conflict of interestNone.
Ethical disclosuresConfidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Protection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals for this investigation.
This work was supported by National Natural Science Foundation of China (No. 81170028) and Shanghai Jiaotong University School of Medicine Fund (No. 12XJ10020).