Corticosteroids are used in the treatment of asthma. The aim of this study was to determine the efficacy of anti-IgE and anti-TNF alpha as asthma treatments.
MethodsA mouse model of chronic asthma was developed. The fluticasone group was exposed to fluticasone and the anti-IgE and anti-TNF groups were administered anti-IgE or anti-TNF. IL-4, and IgE levels were measured, and histological analysis, pathological analysis and miRNA-126, miRNA-133a analyses were applied.
ResultsThe cell concentration in the BAL fluid decreased in all the treatment groups. The rate of perivascular and peribronchial cell infiltration decreased in the lung in the high-dose anti-IgE and anti-TNF groups. Smooth muscle thickness decreased in the lung tissue in the low-dose anti-IgE and anti-TNF groups. Bronchial wall thickness decreased in the lung tissue in the fluticasone+anti-IgE group. The IL-4 level in BAL fluid decreased in the high-dose anti-IgE, fluticasone+anti-IgE and anti-TNF groups. IgE levels increased in the BAL fluid in the high-dose anti-IgE and anti-TNF groups. The lymphocyte level increased in the BAL fluid in the high-dose anti-IgE group. The macrophage level decreased in the BAL fluid in the anti-TNF group. The relative expression of miRNA-126 increased in all groups. The relative expression of miRNA-133a decreased in the placebo and fluticasone groups. The relative expression of miRNA-133a increased in the low-dose anti-IgE, high-dose anti-IgE, fluticasone+anti-IgE and anti-TNF groups.
ConclusionsThe results showed that anti-IgE is successful as a treatment. Fluticasone+anti-IgE and anti-TNF were seen to be superior to other therapeutic modalities when used for prophylaxis.
Asthma is typically a childhood condition characterised by chronic inflammation of the airways.1,2 The most potent of the anti-inflammatory medications used in the treatment of asthma are corticosteroids, with local inhaled steroid therapy preferred due to a more favourable side effect profile. However, inhaled steroids can still have serious, if rarely reported, systemic side effects.1 This possibility of side effects or non-responsiveness to therapy has prompted a continued search for alternative treatments. One alternative agent is anti-IgE, which has been approved for use as an asthma treatment.3
Another possibility is anti-TNF, which has been successfully used in inflammatory diseases such as rheumatoid arthritis and Crohn's disease. The findings from seminal experimental studies using asthmatic Balb/c mice models have been favourable for a role for anti-TNFs in the treatment of chronic asthma.4 Clinical trials using TNF-alpha treatment have been successful in severe and persistent asthma patients.5
The study aim was to evaluate the effects of anti-IgE and anti-TNF alpha treatments on the chronic changes that develop in the lungs of BALB/c mice and to compare these effects with those achieved with inhaled steroids.
Materials and methodsExperimental animalsThe 56 BALB/c mice included in the study were assigned to seven groups, with eight mice per group. The groups were nested as control, placebo, fluticasone, low-dose anti-IgE, high-dose anti-IgE, fluticasone+anti-IgE and anti-TNF groups.
Sensitisation and exposure by inhalationThe chronic asthma model used in this study was that described by Temelkovski et al.6 BALB/c mice were the preferred animal subjects as they have a high IgE response to ovalbumin (OVA) solution.7 The mice in the study groups were sensitised by exposure to 10μg of intraperitoneal chicken egg ovalbumin (OVA; Grade V, Sigma, St Louis, Missouri, USA), which was administered on two occasions 14 days apart (21 and 7 days prior to OVA inhalation). The mice in the control group were administered saline using the same method.8
On the 7th day after immunisation (Day 21), the study groups were exposed to 2.5% OVA solution in aerosolised sterile saline for inhalation for a 30-min duration, three times per week, for a period of eight weeks (Inno Spire Essence; EU Plug/Germany). Mice in the control group were subjected to saline inhalation using the same method.6–8 All animals were sacrificed 48h after the final application.
Drugs used in the studyFluticasone, aerosolised at 2.000μg/2ml, was used for inhalation three times per week.9,10 On the 21st day of the study, anti-IgE was administered intraperitoneally, at 100 or 200μg, at 15-day intervals for a total of five sessions, and anti-TNF at 6.25mg/kg was administered intraperitoneally at the same intervals.11,12
Obtaining BAL fluid and evaluation of IL-4 and IgE levelsA thoracotomy was performed on the 79th day of the study and a 24-gauge cannula connected to the tip of a syringe was introduced into the trachea. Subsequently, 1.2ml of phosphate-buffered saline was administered into the lungs and the cannula was then withdrawn to remove BAL fluid.
The BAL fluid was centrifuged (5min/700rpm) and the supernatant was stored at −70°C until use. The BAL fluid was analysed for IL-4 (Mouse IL-4 ELISA Kit, Boster Biological Technology, Fremont, CA, USA. Detection Limit: 7.8–500pg/ml) and total IgE (Mouse IgE ELISA Kit, Immunology Consultants Laboratory, Portland, OR, USA. Detection Limit: 0.625–40ng/ml) using an ELISA method.11
Cytological evaluations of the BAL fluidThe sediment obtained after centrifugation of the BAL fluid at 700rpm for 5min was spread on a microscope slide and the cells were counted using a light microscope.11 The cell concentration was graded as: 1 (mild), 2 (intermediate) or 3 (intense).
Histopathological analysisTissue samples obtained from the middle zone of the right lungs of all the mice were embedded in paraffin following fixation in 10% formalin and routine histological procedures for light microscope evaluation. Subsequently, serial cross sections 5μm in thickness were cut. For each mouse, 10 sections were selected by randomly selecting the first section and skipping 10 sections in each case, and these were used for staining procedures.
Morphometric evaluations were performed on tissue structures and the thicknesses of subepithelial smooth muscle layers of airways of intermediate and small diameters were determined. Epithelial and subepithelial smooth muscle thickness was measured using a micrometric measurement device that was calibrated at eight different points of three different airways. Peribronchial and perivascular inflammation was evaluated.11 The value of density of inflammation was graded as: 0 (none), 1 (mild), 2 (intermediate) and 3 (intense).
Total RNA isolation and cDNA conversionBoth asthmatic and normal animal tissues were processed using an miRNeasy FFPE Kit (Qiagen GmbH, Hilden, Germany) to obtain total RNA for miRNA expression. The miScript II RT Kit (Qiagen GmbH, Hilden, Germany) was used to obtain cDNA for qRT-PCR analysis. A Rotor-Gene Q (Qiagen Germany) Thermal Cycler was used to reveal the expression of miRNAs. Real-time PCR was performed using the Rotor Gene 6000 Real-Time PCR Machine (Qiagen GmbH, Hilden, Germany) with an miScript SYBR Green PCR Kit (Qiagen GmbH, Hilden, Germany) for miRNA expression.
Statistical analysisThe data were expressed as median and 25–75% percentile values of eight mice. The comparisons of all data were made using the Mann–Whitney test. A value of p<0.05 was accepted as statistically significant.
Statistical analysis of miRNASPSS 22.0 software (SPSS, Chicago, USA) was used for statistical analysis. Relative quantification RT-PCR was performed in triplicate. The 2−ΔΔCT method was used to calculate relative changes. The fold changes in miR-126 and miR-133a were calculated using RT2 Profiler PCR Array Data Analysis version 3.5 (Qiagen GmbH, Hilden, Germany). This analysis program is based on the 2−ΔΔCT method for fold change calculations. Beta actin expression levels were used for normalisation.
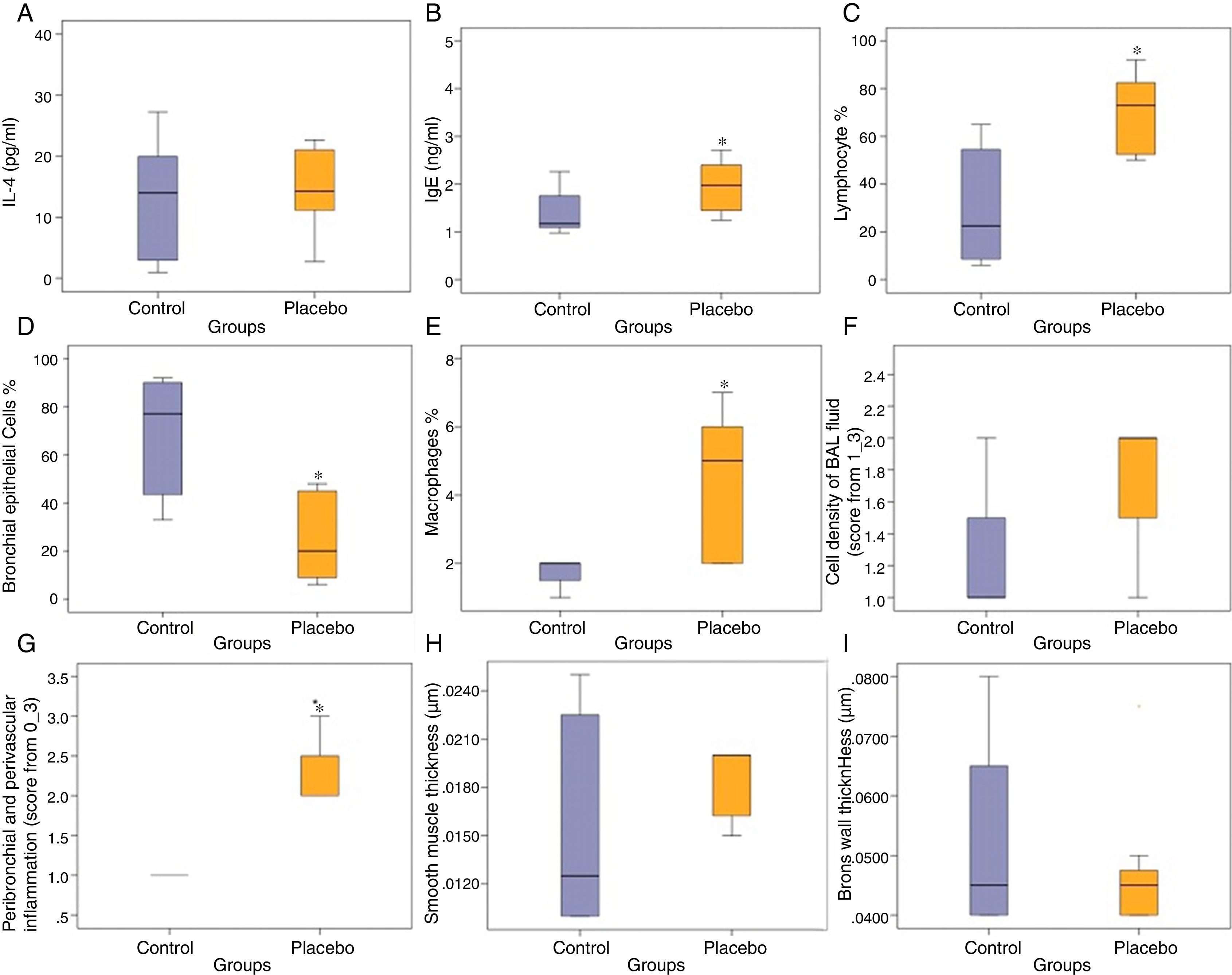
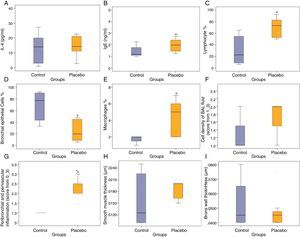
ResultsCompared with the control group, the placebo group had significantly lower bronchial epithelial cells in BAL fluid (p<0.05) (Fig. 1).
Effects of control and placebo on the IL-4, (A) IgE, (B) lymphocyte, (C) bronchial epithelial cells, (D) macrophages, (E) cell density of BAL fluid, (F) peribronchial and perivascular inflammation, (G) smooth muscle thickness, (H) and bronchial wall thickness (I). *P<0.05, compared with the control group (Mann-Whitney U Test).
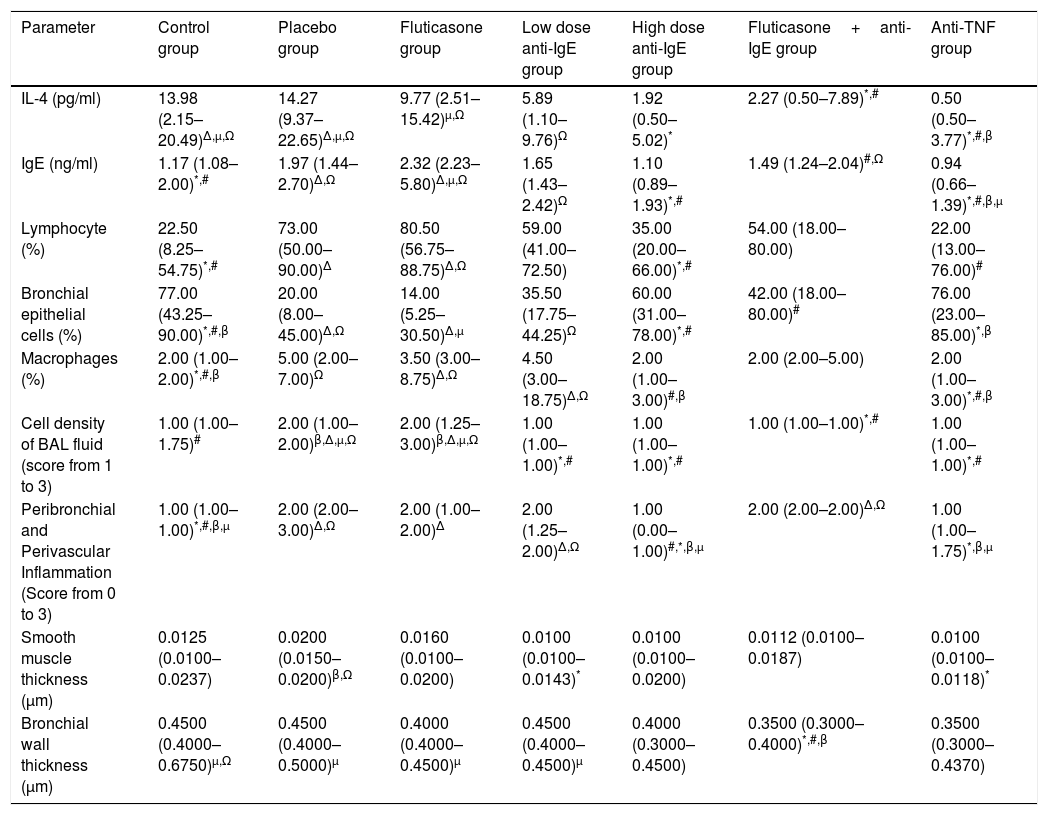
The results of the evaluations of BAL fluid and lung pathology of all the seven groups are shown in Table 1.
Parameters for the control, placebo (asthmatic mice), fluticasone, low dose anti-IgE, high dose anti-IgE, fluticasone+anti-IgE and anti-TNF groups. Data are stated as median (25–75%).
| Parameter | Control group | Placebo group | Fluticasone group | Low dose anti-IgE group | High dose anti-IgE group | Fluticasone+anti-IgE group | Anti-TNF group |
|---|---|---|---|---|---|---|---|
| IL-4 (pg/ml) | 13.98 (2.15–20.49)Δ,μ,Ω | 14.27 (9.37–22.65)Δ,μ,Ω | 9.77 (2.51–15.42)μ,Ω | 5.89 (1.10–9.76)Ω | 1.92 (0.50–5.02)* | 2.27 (0.50–7.89)*,# | 0.50 (0.50–3.77)*,#,β |
| IgE (ng/ml) | 1.17 (1.08–2.00)*,# | 1.97 (1.44–2.70)Δ,Ω | 2.32 (2.23–5.80)Δ,μ,Ω | 1.65 (1.43–2.42)Ω | 1.10 (0.89–1.93)*,# | 1.49 (1.24–2.04)#,Ω | 0.94 (0.66–1.39)*,#,β,μ |
| Lymphocyte (%) | 22.50 (8.25–54.75)*,# | 73.00 (50.00–90.00)Δ | 80.50 (56.75–88.75)Δ,Ω | 59.00 (41.00–72.50) | 35.00 (20.00–66.00)*,# | 54.00 (18.00–80.00) | 22.00 (13.00–76.00)# |
| Bronchial epithelial cells (%) | 77.00 (43.25–90.00)*,#,β | 20.00 (8.00–45.00)Δ,Ω | 14.00 (5.25–30.50)Δ,μ | 35.50 (17.75–44.25)Ω | 60.00 (31.00–78.00)*,# | 42.00 (18.00–80.00)# | 76.00 (23.00–85.00)*,β |
| Macrophages (%) | 2.00 (1.00–2.00)*,#,β | 5.00 (2.00–7.00)Ω | 3.50 (3.00–8.75)Δ,Ω | 4.50 (3.00–18.75)Δ,Ω | 2.00 (1.00–3.00)#,β | 2.00 (2.00–5.00) | 2.00 (1.00–3.00)*,#,β |
| Cell density of BAL fluid (score from 1 to 3) | 1.00 (1.00–1.75)# | 2.00 (1.00–2.00)β,Δ,μ,Ω | 2.00 (1.25–3.00)β,Δ,μ,Ω | 1.00 (1.00–1.00)*,# | 1.00 (1.00–1.00)*,# | 1.00 (1.00–1.00)*,# | 1.00 (1.00–1.00)*,# |
| Peribronchial and Perivascular Inflammation (Score from 0 to 3) | 1.00 (1.00–1.00)*,#,β,μ | 2.00 (2.00–3.00)Δ,Ω | 2.00 (1.00–2.00)Δ | 2.00 (1.25–2.00)Δ,Ω | 1.00 (0.00–1.00)#,*,β,μ | 2.00 (2.00–2.00)Δ,Ω | 1.00 (1.00–1.75)*,β,μ |
| Smooth muscle thickness (μm) | 0.0125 (0.0100–0.0237) | 0.0200 (0.0150–0.0200)β,Ω | 0.0160 (0.0100–0.0200) | 0.0100 (0.0100–0.0143)* | 0.0100 (0.0100–0.0200) | 0.0112 (0.0100–0.0187) | 0.0100 (0.0100–0.0118)* |
| Bronchial wall thickness (μm) | 0.4500 (0.4000–0.6750)μ,Ω | 0.4500 (0.4000–0.5000)μ | 0.4000 (0.4000–0.4500)μ | 0.4500 (0.4000–0.4500)μ | 0.4000 (0.3000–0.4500) | 0.3500 (0.3000–0.4000)*,#,β | 0.3500 (0.3000–0.4370) |
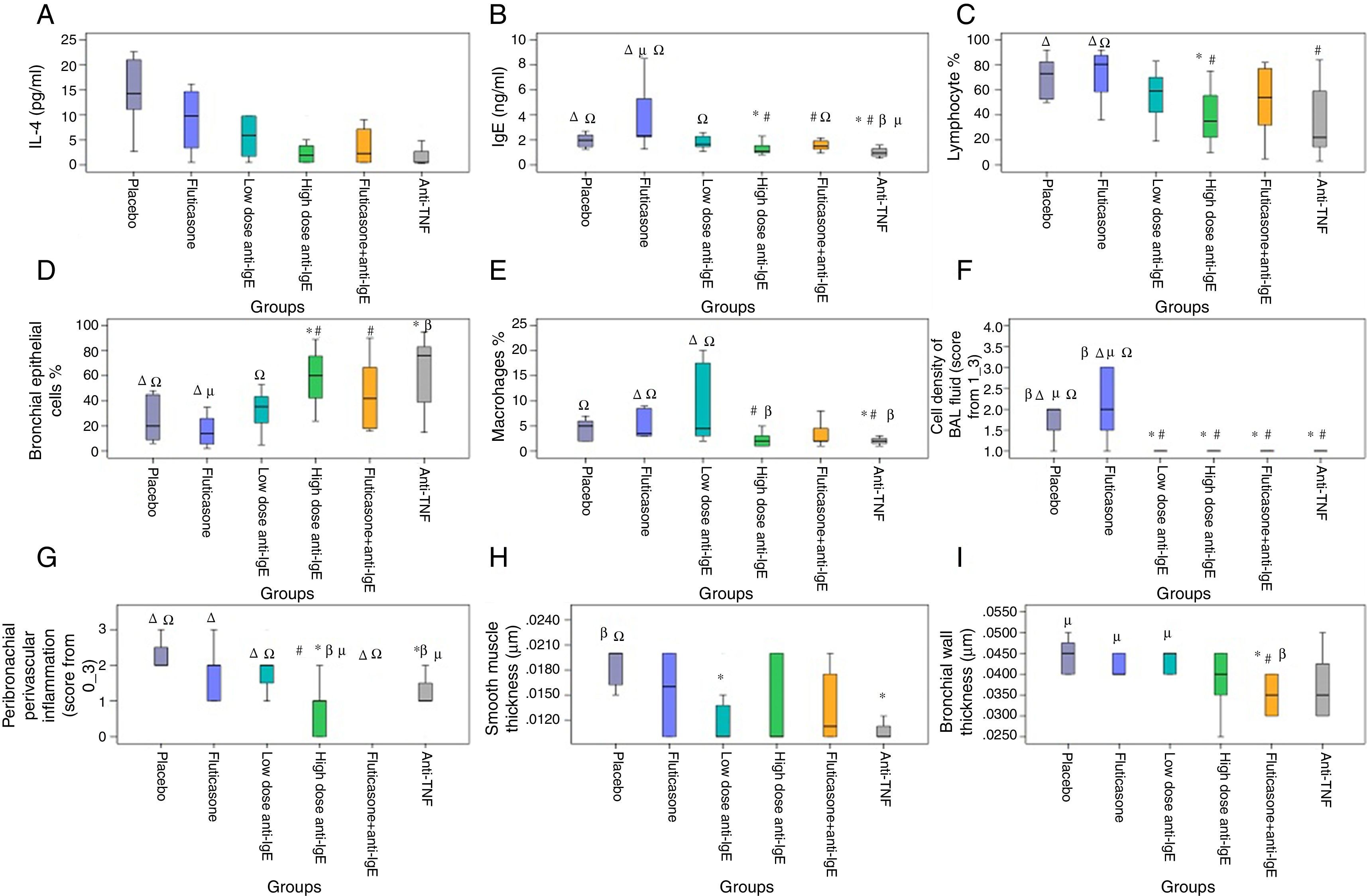
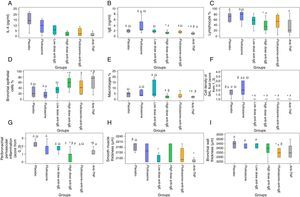
When compared with the placebo group, IL-4 (p=0.006) and IgE (p=0.035) levels decreased in the high-dose anti-IgE group; IL-4 (p=0.005) decreased in the fluticasone+anti-IgE group and IL-4 (p=0.002) and IgE (p=0.011) levels decreased in the anti-TNF group. When compared with the fluticasone group, the IgE level (p=0.074) decreased in the low-dose anti-IgE group; IgE (p=0.008) and IL-4 (p=0.054) levels decreased in the high-dose anti-IgE group; IL-4 (p=0.043) and IgE (p=0.006) levels decreased in the fluticasone+anti-IgE group and IL-4 (p=0.008) and IgE (p=0.002) levels decreased in the anti-TNF group. When compared with the low-dose anti-IgE group, IgE (p=0.083) showed a borderline decrease in the high-dose anti-IgE group, IL-4 (p=0.048) and IgE (p=0.009) levels decreased in the anti-TNF group. When compared with the fluticasone+anti-IgE group, the IgE level (p=0.021) decreased in the anti-TNF group (Fig. 2A and B).
Cytological evaluations of BAL fluidWhen compared with the placebo group, the cell concentration (p=0.023) value in the BAL fluid decreased in the low-dose anti-IgE group; the bronchial epithelium (p=0.018) value increased and the lymphocyte (p=0.029) and cell concentration (p=0.035) levels decreased in the high-dose anti-IgE group; the cell concentration value (p=0.035) in the BAL fluid decreased in the fluticasone+anti-IgE group; the bronchial epithelium value (p=0.025) increased, the cell concentration value (p=0.008) decreased, and the lymphocyte (p=0.064) and macrophage (p=0.062) levels decreased in the anti-TNF group. When compared with the fluticasone group, the cell concentration level (p=0.012) in the BAL fluid decreased in the low-dose anti-IgE group; the bronchial epithelium value (p=0.009) increased, the lymphocyte (p=0.015), macrophage (p=0.023) and cell concentration (p=0.018) levels decreased in the high-dose anti-IgE group; the bronchial epithelium level (p=0.032) increased and the cell concentration (p=0.018) decreased in the fluticasone+anti-IgE group; the bronchial epithelium level (p=0.015) increased and the lymphocyte (p=0.028), macrophage (p=0.004) and cell concentrations (p=0.006) decreased in the anti-TNF group. When compared with the low-dose anti-IgE group, the macrophage value (p=0.026) decreased in the high-dose anti-IgE group, and macrophage value (p=0.008) decreased in the anti-TNF group (Fig. 2C–F).
Histopathological analysisWhen compared with the placebo group, the smooth muscle thickness (p=0.008) decreased in the low-dose anti-IgE group; the peribronchial–perivascular inflammation level (p=0.003) decreased in the high-dose anti-IgE group; the bronchial wall thickness (p=0.007) decreased in the fluticasone+anti-IgE group; and the peribronchial–perivascular inflammation level (p=0.004) and smooth muscle thickness (p=0.001) decreased in the anti-TNF group. When compared with the fluticasone group, the peribronchial–perivascular inflammation level (p=0.024) decreased in the high-dose anti-IgE group; the bronchial wall thickness (p=0.010) decreased in the fluticasone+anti-IgE group; the smooth muscle thickness (p=0.055) and peribronchial–perivascular inflammation level (p=0.094) borderline decreased in the anti-TNF group. When compared with the low-dose anti-IgE group, the peribronchial–perivascular inflammation level (p=0.012) decreased in the high-dose anti-IgE group; the bronchial wall thickness (p=0.004) decreased in the fluticasone+anti-IgE group; the peribronchial–perivascular inflammation level (p=0.039) decreased and the bronchial wall thickness (p=0.064) was in the anti-TNF group. When compared with the high-dose anti-IgE group, the peribronchial–perivascular inflammation level (p=0.002) decreased in the anti-IgE+fluticasone group. When compared with the fluticasone+anti-IgE group, the peribronchial–perivascular inflammation level (p=0.003) decreased in the anti-TNF group (Fig. 2G–I).
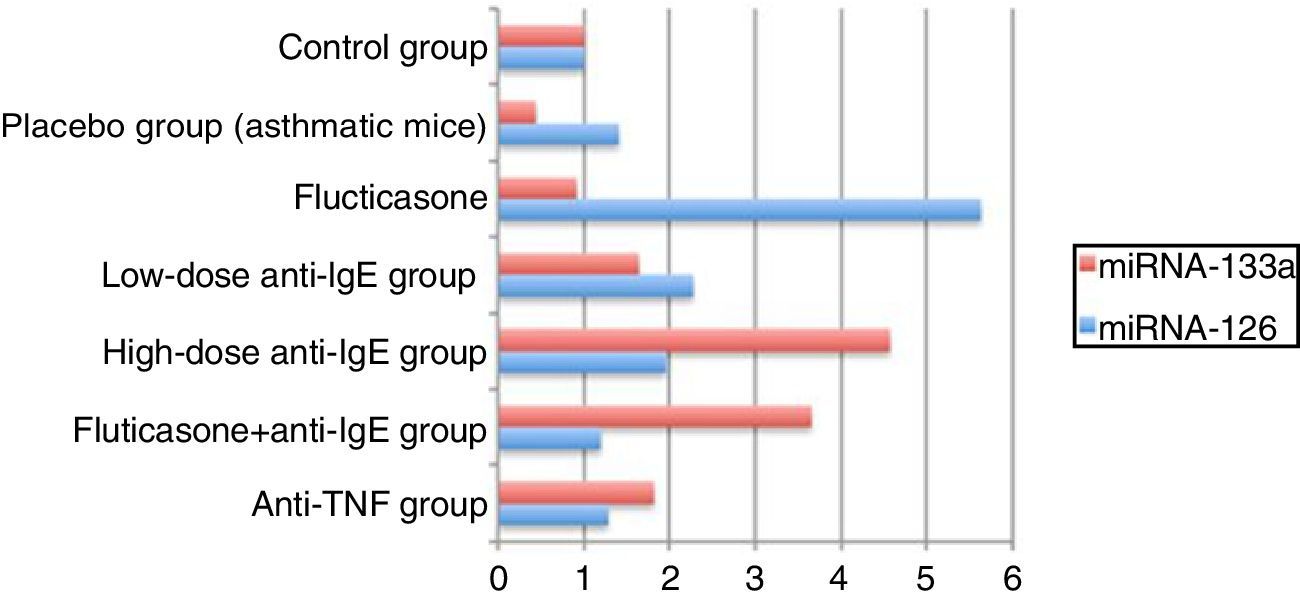
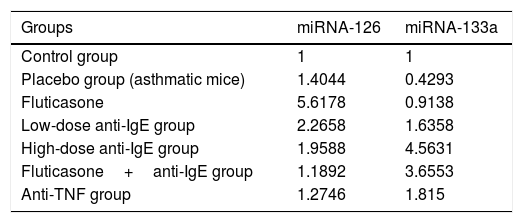
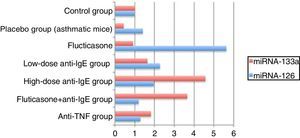
miRNA analysis in the lung tissueThe expression values of miRNA-126 and miRNA-133a measured in the control group were assigned a value of 1 and the expression values for mice in the other groups were calculated relative to this control value. Compared to the control group, the placebo group, which did not receive treatment, demonstrated an increase in miRNA-126 relative expression to a value of 1.4044, while the relative expression of miRNA-133a decreased, calculated at 0.4293 compared to the control. In the treatment groups, the relative expression of miRNA-126 compared to the control group was, in order: fluticasone group=5.6178; low-dose anti-IgE group=2.2658; high-dose anti-IgE group=1.9588; fluticasone+anti-IgE group=1.1892; and anti-TNF group=1.2746. In the treatment groups, the relative expression of miRNA-133a compared to the control group was, in order: fluticasone group=0.9138; low-dose anti-IgE group=1.6358; high-dose anti-IgE group=4.5631; fluticasone+anti-IgE group=3.6553; and in the anti-TNF group=1.8150 (Table 2).
Relative expression values for miRNA-126 and miRNA-133a in the control, placebo (asthmatic mice), fluticasone, low-dose anti-IgE, high-dose anti-IgE, fluticasone+ anti-IgE and anti-TNF groups.
| Groups | miRNA-126 | miRNA-133a |
|---|---|---|
| Control group | 1 | 1 |
| Placebo group (asthmatic mice) | 1.4044 | 0.4293 |
| Fluticasone | 5.6178 | 0.9138 |
| Low-dose anti-IgE group | 2.2658 | 1.6358 |
| High-dose anti-IgE group | 1.9588 | 4.5631 |
| Fluticasone+anti-IgE group | 1.1892 | 3.6553 |
| Anti-TNF group | 1.2746 | 1.815 |
The findings in the present study indicated that inhaled fluticasone application is not sufficient to be able to reverse the chronic histological changes occurring in this asthma model. In contrast, anti-IgE treatment was less effective at low doses, but it reversed these changes at high doses. The study also provided evidence of a higher efficiency of the combined use of fluticasone and low-dose anti-IgE treatment compared to the use of each drug alone. Anti-TNF use alone was more successful in reversing all the chronic changes when compared to the other treatment methods.
Cytokine and IgE analysis in the BAL fluidMcMillan et al.13 evaluated the IL-4 level in the lung tissue of rats and demonstrated a decrease in the IL-4 level in the group receiving budesonide as an inhaled steroid. Kang et al.11 demonstrated a significant decrease in IL-5 in the BAL fluid of rats administered anti-IgE. In the present study, the IL-4 level in the BAL fluid in the group that was administered low-dose anti-IgE was suppressed to an almost statistically significant level when compared to placebo. The IL-4 and IgE levels in the BAL fluid in the group administered high-dose anti-IgE decreased compared to the placebo group. This result reflects the dose-dependent effect of anti-IgE. IL-4 level decreased in the fluticasone+anti-IgE group compared to the placebo group. Although the anti-IgE used was low dose, no statistically significant difference was found in the IL-4 level between the inhaled steroids and low-dose anti-IgE treatments compared to placebo, while the combined use of these two drugs provided a statistically significant decrease in the IL-4 level. This result emphasises the importance of combined treatments.
Deveci et al.12 applied anti-TNF at doses of 2.5mg/kg and 6.25mg/kg in two different groups. Whilst no difference was found in the IL-4 levels between the low-dose anti-TNF group and the placebo group, the IL-4 level was significantly lower in the high-dose anti-TNF group. In the present study, IL-4 and IgE levels decreased in the anti-TNF group compared to the placebo group. Similar results to those reported by Deveci et al.,12 who applied high-dose anti-TNF, were found in this present study following application of high-dose anti-TNF.
While no difference in IL-4 and IgE levels was found between the fluticasone and low-dose anti-IgE groups, the IgE level decreased in the high-dose anti-IgE group. This result demonstrates that the anti-IgE effect is dose-dependent. The IL-4 and IgE levels decreased in the fluticasone+IgE group compared to the fluticasone group, which emphasises the efficacy of combined treatment. The demonstration of decreased levels of IL-4 and IgE in the anti-TNF group compared to the fluticasone group suggests that anti-TNF administration is more efficacious as an asthma treatment than the steroids, which are the major drugs in current use for the treatment of asthma.
The demonstration of decreased levels of IL-4 and IgE in the anti-TNF group compared to the low-dose anti-IgE group and the decreased level of IgE in the anti-TNF group compared to the fluticasone+anti-IgE group suggest that anti-TNF administration is more efficacious than anti-IgE use.
Cytological analysis of the BAL fluidThe important role of lymphocytes and macrophages was emphasised in a rat asthma model in a study by Heialy et al.14 The cell concentration value was reported to be increased in the placebo group in a study by Riesenfeld et al.9 In the present study, the lymphocyte and macrophage levels increased and the bronchial epithelium value decreased in the placebo group. Kang et al.11 demonstrated a decrease in the total cell count in the BAL fluid in response to the anti-IgE treatment. In the present study, the cell concentration value in the BAL fluid decreased in the low-dose anti-IgE group compared to the placebo group, while the bronchial epithelium value increased and the lymphocyte and cell concentration levels decreased in the high-dose anti-IgE group. This result suggests that the efficacy of the anti-IgE treatment decreases with increasing dosage. When compared with the placebo group, the fluticasone+anti-IgE group showed a decreased cell concentration in the BAL fluid. This result indicates that a combined treatment is more efficacious in decreasing the cell concentration in BAL tissue. In addition, the bronchial epithelium value increased and the cell concentration value decreased in the BAL fluid in the anti-TNF group when compared to the placebo group. This result suggests that treatment with anti-TNF is efficacious at decreasing the cell concentration in the BAL fluid.15
Payne et al.16 stated that current asthma drugs suppressed inflammation, although these drugs were found to be ineffective for remodelling. In the present study, the cell concentration level in the BAL fluid decreased in the anti-IgE group compared to the fluticasone group, which is similar to the results published by Payne et al.16 In addition, the bronchial epithelium level increased and lymphocyte, macrophage and cell concentration levels decreased in the high-dose anti-IgE group compared to the fluticasone group. This result, in contrast, reflects the dose dependence of the anti-IgE effect, as the effect increases with increasing dose. The bronchial epithelium level also increased and the cell concentration value decreased in the BAL fluid in the fluticasone+anti-IgE group compared to the fluticasone group, indicating that combined treatment is more efficacious when compared to inhaled steroid treatment alone. Furthermore, the bronchial epithelium value increased and the lymphocyte, macrophage and cell concentration values decreased in the BAL fluid in the anti-TNF group when compared to the fluticasone group. These results demonstrate that treatment with anti-TNF is quite efficacious in terms of decreasing the lymphocyte, macrophage and cell concentrations in BAL fluid.
Histopathological analysis of the lung tissueRiesenfeld et al. reported increased peribronchial and perivascular inflammation values in an asthmatic group compared to the control group.9 Kang et al. found that smooth muscle thickness decreased in a low-dose anti-IgE group when compared to the placebo group.11 The results of the present study were similar to these previously reported findings, as anti-IgE treatment had positive effects on remodelling. The bronchial wall thickness decreased in the fluticasone+anti-IgE group compared to the placebo group in the present study, suggesting that a combined treatment with fluticasone+anti-IgE is particularly effective in remodelling. The peribronchial–perivascular inflammation and smooth muscle thickness values decreased in the anti-TNF group compared to the placebo group, suggesting that the anti-TNF treatment was also effective in remodelling. No difference was noted for the anti-IgE group compared to the fluticasone group, but the peribronchial–perivascular inflammation levels decreased in the high-dose anti-IgE group, indicating that anti-IgE treatment is more efficacious at high doses compared to inhaled steroids. The bronchial wall thickness also decreased in the fluticasone+anti-IgE group compared to the fluticasone group, suggesting that the combined use of fluticasone+anti-IgE treatment is more efficacious than the use of inhaled steroids alone. Conversely, the observation of decreased peribronchial–perivascular inflammation values in the high-dose anti-IgE group compared to the low-dose anti-IgE group suggested that anti-IgE treatment is more efficacious at high doses due to its dose dependence. The decreased bronchial wall thickness in the fluticasone+anti-IgE group compared to the low-dose anti-IgE group suggested that the combined use of fluticasone+anti-IgE was more efficacious than anti-IgE use alone.
The peribronchial–perivascular inflammation value decreased in the anti-TNF group compared to the low-dose anti-IgE group, while no statistically significant difference was found between the groups when the high-dose anti-IgE group and anti-TNF group were compared. These results also confirm the dose dependence of the anti-IgE treatment and its greater efficacy at high doses. The peribronchial–perivascular inflammation value also increased in the fluticasone+anti-IgE group compared to the high-dose anti-IgE group, suggesting that treatment with high-dose anti-IgE was more efficacious than the combined treatment with fluticasone+low-dose anti-IgE. The peribronchial–perivascular inflammation values decreased in the anti-TNF group compared to the fluticasone+anti-IgE group.
miRNA analysis in the lungsThe comparison of the control group with the asthmatic (placebo) group demonstrated a 1.4-fold increase in the relative miRNA-126 expression value and a 0.4-fold decrease in the relative miRNA-133a expression value, indicating adequate stimulation of the chronic asthma model.16,17 A lower relative expression of miRNA-126 was expected in the fluticasone treatment group than in the placebo group but, contrary to this expectation, a 5.6-fold increase was measured. A clinical trial, in which one group of asthma patients received a placebo and the other group were given inhaled steroids, reported a 5-fold greater expression of miRNA-126 in the placebo group than in the control group, while the group receiving steroid therapy showed a 2-fold increase in miRNA-126 expression.5 These results are comparable to the results in the current study. Furthermore, the comparison of miRNA-133a relative expression in the fluticasone treatment group with that of the placebo group revealed a decrease in the recorded values. Despite this difference, a relative expression value of no less than 0.9 was recorded. In previous studies, low miRNA-133a expression values have been correlated with a decreased incidence of asthma.4
These results indicate that fluticasone is unsuccessful in prophylaxis, while being moderately successful as a therapeutic agent. When compared to the placebo group, the low-dose anti-IgE treatment increased the miRNA-126 relative expression. The favourable results obtained in the treatment groups are consistent with the results of earlier clinical trials, but the values still indicate an insufficient response to fluticasone treatment.5 However, in the low-dose anti-IgE treatment, when compared to the placebo group, successful suppression was indicated by the relative expression values for miRNA-133, which even showed a 1.6-fold increase. According to these results, low-dose anti-IgE treatment is unsuccessful in prophylaxis, but has good success in treatment. It has been reported in literature that miRNA-133a can be used as a target marker for asthmatic bronchial hyperactivity.18
The high-dose anti-IgE-treated group showed relative miRNA-126 expression comparable to the placebo group, while the low-dose anti-IgE group displayed a 1.9-fold increase, instead of a decrease in relative expression. When the placebo group was compared to the high- and low-dose anti-IgE groups in regard to the relative miRNA-133 expression, the measurements were significantly higher for the high-dose anti-IgE group than for the low-dose IgE group. This effect was approximately 10-fold greater than that of the placebo group, and 4.5- and 3-fold higher than the fluticasone and low-dose anti-IgE-treated groups, respectively. According to these results, it was concluded that high-dose anti-IgE treatment is unsuccessful in prophylaxis, but is very effective when used as a therapeutic agent, as the most effective therapy results were seen in the high-dose anti-IgE group. Indeed, the use of high-dose anti-IgE has become more widespread, as it seems to be the most effective in targeting miRNA-133a expression.18
Groups receiving the fluticasone+anti-IgE treatment showed decreased relative expression of miRNA-126 when compared to the placebo group. However, this treatment was unable to prevent 1.1-fold increases when compared with the control group. Nevertheless, the most effective treatment in preventing the increase in miRNA-126 relative expression was the Fluticasone+anti-IgE treatment. Therefore, this combination therapy would be the most successful for prophylaxis. According to the literature, the prevention of asthma is directly related to the prevention of miRNA-126 expression.16,17 In agreement with these results, the combination fluticasone+anti-IgE therapy increased the relative expression of miRNA-133a when compared to the placebo group. However, it was not as effective as the high-dose anti-IgE treatment, which gave a result approximately 8-fold that of the placebo, 4-fold that of the fluticasone group, and 2.2-fold that of the low-dose IgE group. According to the results of this study, the second-best treatment was fluticasone+anti-IgE.18 Those receiving anti-TNF therapy showed a decreased expression of miRNA-126 relative to the placebo group, but TNF treatment was unable to prevent a 1.2-fold increase compared to the control group. Nevertheless, the anti-TNF treatment was the second-best treatment for the prevention of miRNA-126 relative expression. In other words, the anti-TNF treatment was the second best for prophylaxis.16,17 Furthermore, when the anti-TNF group was compared with the placebo group, the relative 1.8-fold increase in the miRNA-133a expression value made it the third-best treatment, behind the high-dose anti-IgE and fluticasone+anti-IgE treatments. Taking all these results into consideration, the optimal choice for both treatment and prophylaxis is the fluticasone+anti-IgE combination therapy17,18 (Fig. 3).
Compliance with inhaled corticosteroid medical therapy for many asthmatics is suboptimal due to patient attitudes regarding the side effects of steroids.19 Furthermore, some patients fare poorly despite intensive therapy and are classified as relatively steroid insensitive.20 Circumvention of some of the problems in treatment, remediation of the chronic changes that occur in the lungs and increasing the success in asthma control will require continued research into alternative therapies (preferably non-steroidal in nature).21 The current study proposes anti-IgE and anti-TNF alpha as two such treatments, as they show superior performance to inhaled steroid treatment.
In conclusion, fluticasone treatment was unsuccessful both as prophylaxis and therapy. Low-dose anti-IgE and high-dose anti-IgE therapies were unsuccessful in prophylaxis, but were successful and have a role in therapy. The most successful therapy was high-dose anti-IgE. Fluticasone+anti-IgE and anti-TNF, while superior in prophylaxis, were only moderately successful in treatment. When all the results are considered, a combined fluticasone+anti-IgE therapy was seen to produce ideal results for both prophylaxis and treatment.
Ethical statementsEthical approval was obtained from University of Gaziantep.
Funding sourceGaziantep University Scientific Research Projects.
Conflicts of interestThe authors have no conflicts of interest regarding the content of this article.
All authors contributed to designing the study, collecting and analysing the data, writing, and revising the manuscript.