In contrast to adult asthmatic patients, studies on the role of serum periostin levels in schoolchildren with asthma are still conflictive, and very few studies have been performed in pre-schoolers. The aim of this study was to compare serum periostin levels in recurrent wheezer pre-schoolers according to their asthma predictive index (API) condition.
MethodsWe performed a case–control study enrolling pre-schoolers with recurrent wheezing episodes (>3 episodes confirmed by physician) presented at one paediatric clinic in Santiago, Chile. The population was divided according to stringent API criteria into positive or negative.
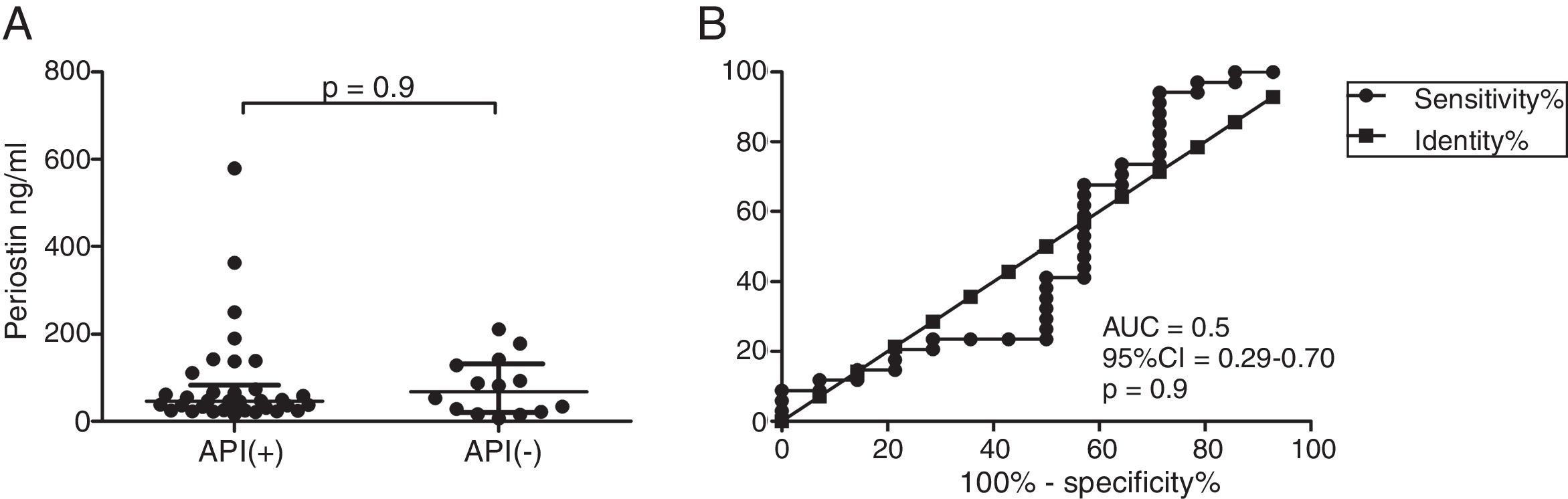
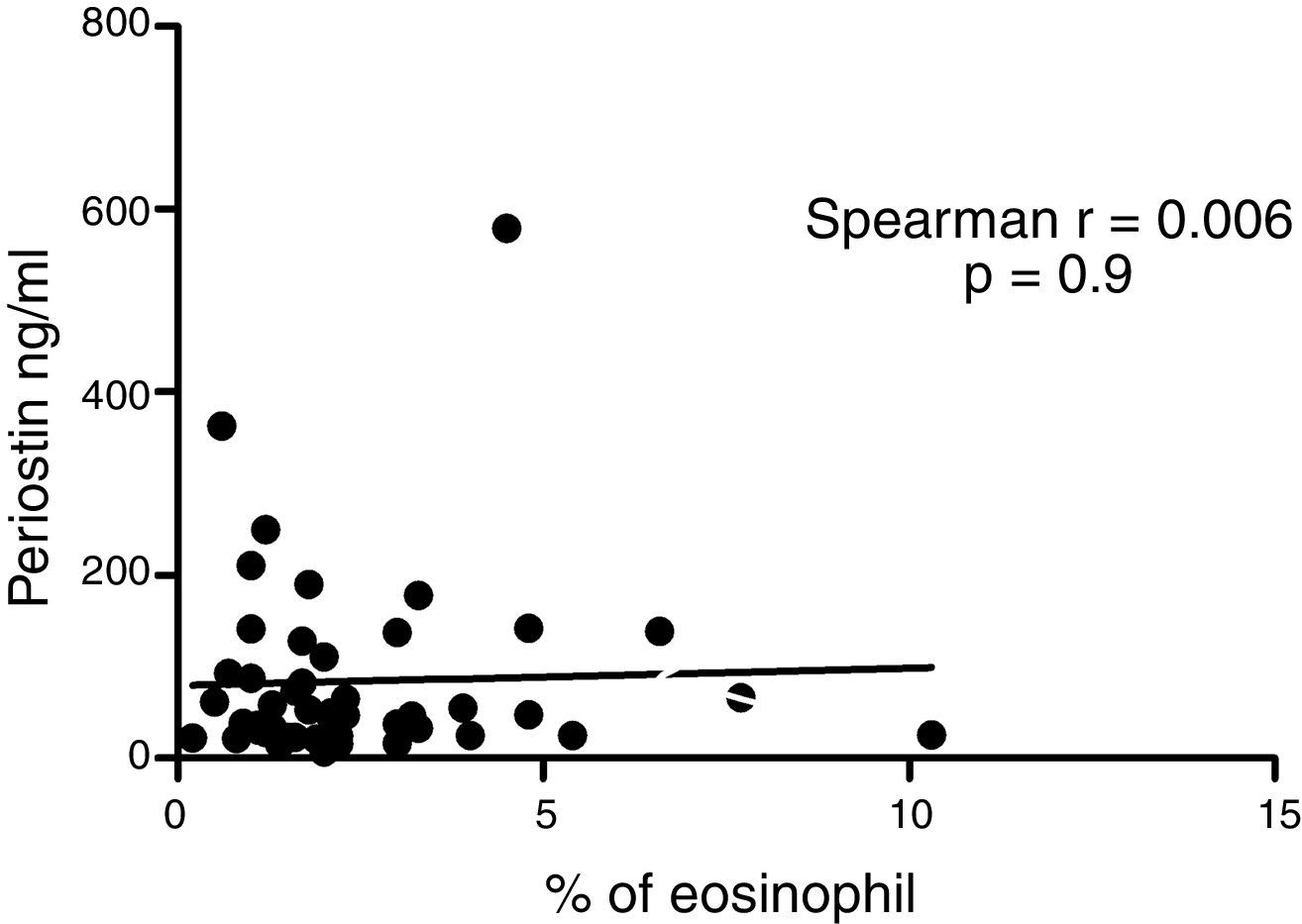
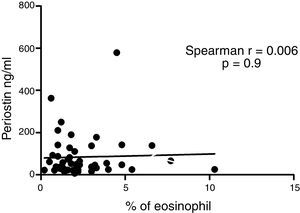
ResultsIn a one-year period, 60 pre-schoolers were enrolled. After excluding 12 (due to not fulfilment of inclusion criteria or refusal of blood sample extraction), 48 remaining pre-schoolers (27 males, age range from 24 to 71 months) completed the study; 34 were API positive and 14 were API negative. There were no significant differences in demographics between groups. The level of serum periostin levels for pre-schoolers with positive API and negative API were (median 46.7 [25.5–83.1] and 67.5 [20.5–131.8], p=0.9, respectively). The area under the curve for the serum periostin levels for predict positive API was 0.5, 95% CI [0.29–0.70], p=0.9. No significant correlation between serum periostin levels and peripheral blood eosinophils was found.
ConclusionSerum periostin levels were no significantly different between wheezer pre-schoolers with positive and negative API. More studies are needed to confirm this finding.
Asthma is one of the most common chronic diseases in children.1 Recurrent wheezing in pre-school age is frequently the presenting sign of asthma; however, many children who wheeze during early life do not go on to develop childhood asthma.1 Based on the epidemiological data on the natural history and temporal patterns of wheezing, several childhood wheezing phenotypes have been described. However, the use of these “epidemiological” phenotypes of wheezing is limited, since they can only be identified retrospectively; indeed, they were defined using statistical inference on longitudinally collected data, and are not useful in the present as they are defined by events that will occur in the future.2
To solve that problem, in the past decades, several asthma predictive rules have emerged.3 The Asthma Predictive Index (API)4 uses only clinical parameters (paternal asthma, rhinitis, dermatitis, wheezing without cold and eosinophils in peripheral blood) is the most widely used and the only one that fulfils all the steps for clinical prediction rules,5 e.g. development,4 validation/updating,6–8 impact9–12 and implementations.13–16 The API which is related with atopic asthma inception is simple and cheap, and its major strength is its good positive likelihood ratio ∼7.4 and high specificity (∼97%).17
Peripheral blood eosinophils have been identified as a surrogate marker of type 2 inflammation. These particular cells are considered the main effector cell in the pathophysiology of asthma.18 High levels of peripheral blood eosinophils are recognised as an important biomarker for the eosinophilic asthma phenotype and have been identified as a readily available biomarker that correlates with disease severity and may predict response to asthma therapy.19,20
Periostin is a matricellular protein whose expression can be induced by type 2 inflammatory cytokines IL-4 and IL-13, as well as by other stimuli such as TGF-beta. IL-13 induces the secretion of periostin from bronchial epithelial cells.21 Periostin is secreted basally from airway epithelial cells where it has pleotropic effects on epithelial cell function and on the development of airway fibroblasts, which is thought to promote airway remodelling in asthmatic patients. An in vitro study showed that airway epithelial cells from schoolchildren with allergic asthma express higher levels of periostin than cells from children without asthma.22 Periostin levels in peripheral blood have been identified as an easily obtained systemic biomarker of type 2 airway inflammation in adults and may also predict responsiveness to therapy (e.g. inhaled corticosteriods, lebrikizumab).21,23
However, the literature on serum periostin levels in asthmatic children is scarce and conflictive. For example, one study on schoolchildren suggests that elevated serum periostin level correlates with airway hyperresponsiveness, whereas another found that high periostin level was not a predictor of asthma morbidity.24,25 Moreover, only one study was performed at pre-school age reporting that those who developed asthma by age six years had increased serum periostin at age two years, in comparison to those who did not develop asthma by age six years.26
The objective of this study is to assess if pre-schoolers with recurrent wheezing episodes and positive API differ in serum periostin levels than those with negative API, and if peripheral eosinophils correlate with serum periostin levels in this particular age group. Our hypothesis is that pre-schoolers with positive API would have higher serum periostin levels than those with negative API.
MethodsIn this case–control study, a convenience sample of sixty-two pre-schoolers (2–5 years of age) with recurrent wheezing episodes (more than three episodes/year) who consulted in the outpatient paediatric clinic at Pontificia Universidad Catolica de Chile were recruited from March 2015 to March 2016. The study was previously approved by the Ethical Committee at the Pontificia Universidad Catolica de Chile (# 14-048). Informed consent was obtained from the parents or legal guardians before patient inclusion.
The inclusion criteria were: recurrent wheezing (≥3 episodes per year with diagnosis by a paediatrician) without inhaled corticosteroids (ICS) treatment in the last month and without leukotriene receptor antagonist (LTRA) in the last two weeks. Exclusion criteria were patients with other chronic respiratory illness, e.g. cystic fibrosis, bronchopulmonary dysplasia, post-infection bronchiolitis obliterants, cardiac or pulmonary malformations, and acute respiratory infection in the last three weeks.
A detailed questionnaire was completed and collected at enrolment. It included demographic characteristics, parental history of asthma and allergic diseases, rhinitis, atopic dermatitis, history of wheezing episodes, onset and their severity, cough at night and after exercise, parental higher educational degree, exposure to tobacco smoke, and day-care attendance. On the same day, a peripheral blood sample was obtained to assess eosinophil counts and serum periostin levels. Serum was stored at −20°C before measurement of periostin level. Periostin was determined by ELISA (Human Periostin/OSF-2 ELISA Kit, Wuhan City, China) and expressed as ng/mL.
Using the data from the questionnaire and peripheral blood eosinophils count, patients were divided into two groups: positive API and negative API. We used the stringent API definition, e.g. positive API was considered if they had one major (parental MD asthma or MD eczema) or two minor criteria (MD allergic rhinitis, wheezing apart from colds or peripheral blood eosinophilia >4%).4
Statistical analysisWe compared pre-schoolers with positive API versus negative API. Continuous variables with normal distribution were described in mean±standard deviation (SD), and compared using T-test. Continuous variables without normal distribution were described in median [25–75 percentile], and used non-parametric test (Mann–Whitney). Categorical variables were described in contingency tables and compared using chi2 with Fisher exact test. A bivariate risk analysis was performed by calculating odds ratio (OR) with 95% confidence interval (CI). Spearman's rho correlation between the serum periostin levels and peripheral blood eosinophils was performed. The receiver operational curve (ROC) analyses were performed to investigate the capacity of serum periostin levels to predict positive API. The overall accuracy of the test was measured as the area under the ROC curve. Statically significant differences were considered for a p value<0.05. All statistical analyses were performed using GraphPad Prism V.5.
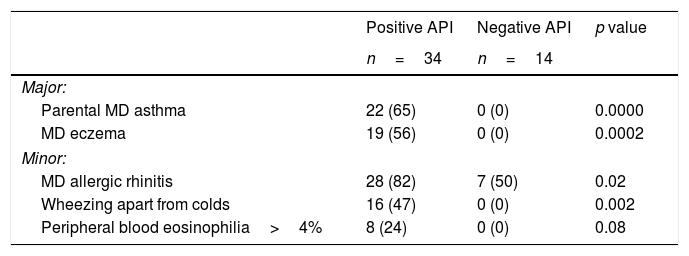
ResultsSixty pre-schoolers were initially enrolled, 12 declined to participate (mainly because they did not fulfil inclusion criteria or refusal of blood sample extraction). Of the remaining 48 pre-schoolers, 27 (56%) were males, the age range was 24–71 months, 34 had positive API, and 14 had negative API. The distribution of major and minor API criteria between children with positive and negative API are shown in Table 1.
Presence of major and minor criteria of asthma predictive index between groups.
| Positive API | Negative API | p value | |
|---|---|---|---|
| n=34 | n=14 | ||
| Major: | |||
| Parental MD asthma | 22 (65) | 0 (0) | 0.0000 |
| MD eczema | 19 (56) | 0 (0) | 0.0002 |
| Minor: | |||
| MD allergic rhinitis | 28 (82) | 7 (50) | 0.02 |
| Wheezing apart from colds | 16 (47) | 0 (0) | 0.002 |
| Peripheral blood eosinophilia>4% | 8 (24) | 0 (0) | 0.08 |
API=asthma predictive index; MD=medical diagnosis.
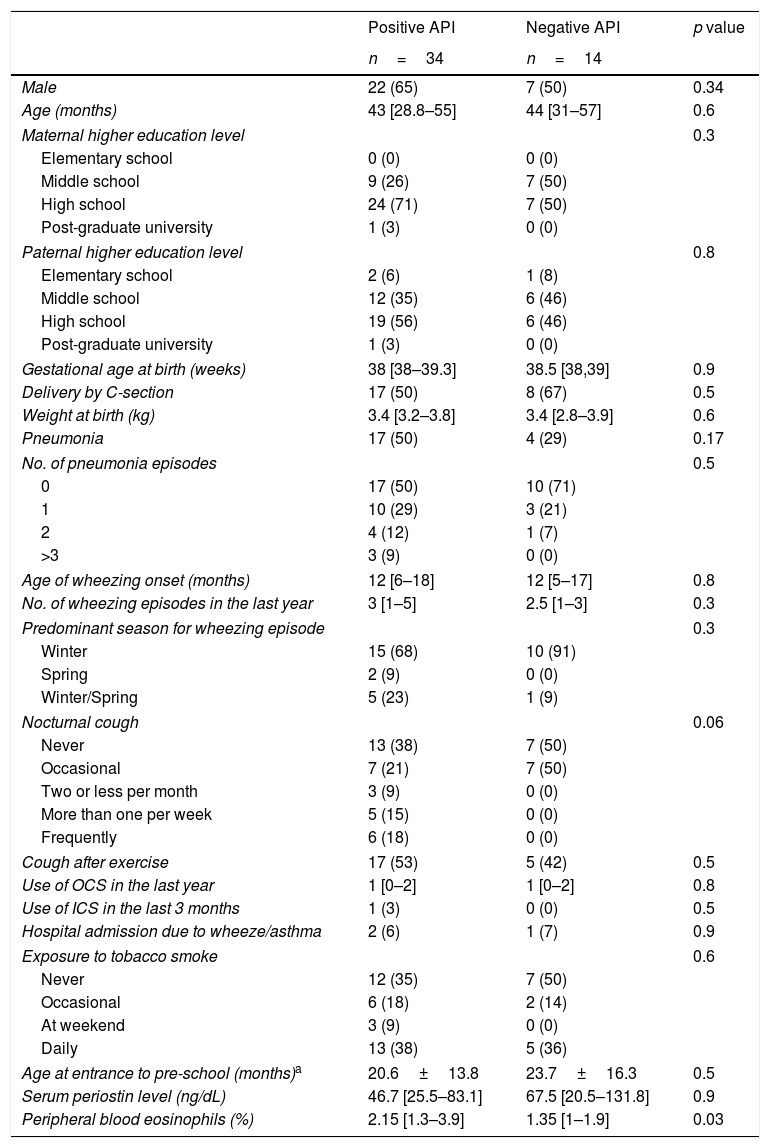
There were no significant differences in terms of demographic and perinatal characteristics, maternal education, day-care entrance, and tobacco exposure between those patients with positive and negative API (Table 2). Pneumonia was more prevalent in the positive than negative API group (50% vs. 29%, p=0.17). Also, no significant difference was observed in terms of wheezing episodes characteristics (age of onset, seasonality, oral steroids burst, and hospitalisations). Pre-schoolers with positive API had more cough at night than negative API patients (62 vs. 59%, p=0.06, respectively). Only 3% of pre-schoolers with positive API and none in the negative API group received ICS in the past as part of their management (Table 2).
Demographic and clinical characteristic of pre-schoolers with positive and negative asthma predictive index.
| Positive API | Negative API | p value | |
|---|---|---|---|
| n=34 | n=14 | ||
| Male | 22 (65) | 7 (50) | 0.34 |
| Age (months) | 43 [28.8–55] | 44 [31–57] | 0.6 |
| Maternal higher education level | 0.3 | ||
| Elementary school | 0 (0) | 0 (0) | |
| Middle school | 9 (26) | 7 (50) | |
| High school | 24 (71) | 7 (50) | |
| Post-graduate university | 1 (3) | 0 (0) | |
| Paternal higher education level | 0.8 | ||
| Elementary school | 2 (6) | 1 (8) | |
| Middle school | 12 (35) | 6 (46) | |
| High school | 19 (56) | 6 (46) | |
| Post-graduate university | 1 (3) | 0 (0) | |
| Gestational age at birth (weeks) | 38 [38–39.3] | 38.5 [38,39] | 0.9 |
| Delivery by C-section | 17 (50) | 8 (67) | 0.5 |
| Weight at birth (kg) | 3.4 [3.2–3.8] | 3.4 [2.8–3.9] | 0.6 |
| Pneumonia | 17 (50) | 4 (29) | 0.17 |
| No. of pneumonia episodes | 0.5 | ||
| 0 | 17 (50) | 10 (71) | |
| 1 | 10 (29) | 3 (21) | |
| 2 | 4 (12) | 1 (7) | |
| >3 | 3 (9) | 0 (0) | |
| Age of wheezing onset (months) | 12 [6–18] | 12 [5–17] | 0.8 |
| No. of wheezing episodes in the last year | 3 [1–5] | 2.5 [1–3] | 0.3 |
| Predominant season for wheezing episode | 0.3 | ||
| Winter | 15 (68) | 10 (91) | |
| Spring | 2 (9) | 0 (0) | |
| Winter/Spring | 5 (23) | 1 (9) | |
| Nocturnal cough | 0.06 | ||
| Never | 13 (38) | 7 (50) | |
| Occasional | 7 (21) | 7 (50) | |
| Two or less per month | 3 (9) | 0 (0) | |
| More than one per week | 5 (15) | 0 (0) | |
| Frequently | 6 (18) | 0 (0) | |
| Cough after exercise | 17 (53) | 5 (42) | 0.5 |
| Use of OCS in the last year | 1 [0–2] | 1 [0–2] | 0.8 |
| Use of ICS in the last 3 months | 1 (3) | 0 (0) | 0.5 |
| Hospital admission due to wheeze/asthma | 2 (6) | 1 (7) | 0.9 |
| Exposure to tobacco smoke | 0.6 | ||
| Never | 12 (35) | 7 (50) | |
| Occasional | 6 (18) | 2 (14) | |
| At weekend | 3 (9) | 0 (0) | |
| Daily | 13 (38) | 5 (36) | |
| Age at entrance to pre-school (months)a | 20.6±13.8 | 23.7±16.3 | 0.5 |
| Serum periostin level (ng/dL) | 46.7 [25.5–83.1] | 67.5 [20.5–131.8] | 0.9 |
| Peripheral blood eosinophils (%) | 2.15 [1.3–3.9] | 1.35 [1–1.9] | 0.03 |
Numbers are expressed as (%), mean±SD, or median [25–75 percentile] when corresponding.
Serum periostin levels were not significantly different between pre-schoolers with positive and negative API (46.7 [25.5–83.1]ng/dL vs. 67.5 [20.5–131.8], p=0.9 respectively) (Table 2 and Fig. 1A). The AUC for the serum periostin levels for predicting positive API was 0.5, 95% CI [0.29–0.70], p=0.9 (Fig. 1B). No significant correlation between serum periostin levels and peripheral blood eosinophils was found (Fig. 2).
Serum periostin levels in pre-schoolers are not able to discriminate between pre-schoolers with differential API score. (A) Serum periostin levels (ng/mL) in pre-schoolers with positive and negative API. Data are presented as individual patient's points plus median and 25–75 percentile. Analysis was performed using Mann–Whitney test. (B) Receiver operating curve (ROC) analysis for periostin levels and API Score. AUC is shown.
This study on 48 recurrent wheezing Chilean pre-schoolers showed, contrary to our hypothesis, no significant difference in periostin serum levels between positive or negative API patients. To our knowledge, this is the first study that compares periostin serum levels of pre-schoolers classified by the presence of asthma predictive rule.
The other only study done in pre-schoolers26 using a selected population (high risk for the development of asthma and allergic disease) found that serum periostin levels were significantly higher in children than in adults, likely due to bone turnover occurred in childhood. The levels were highest at two years (145ng/mL), and did not change significantly between four to 11 years (128 and 130ng/mL). At age two years, periostin level of ≥150ng/mL predicted asthma at age six years (OR: 2.3 [1.3–4.4]), but not at 11 years (OR: 1.0 [0.5–1.9]).
Moreover, studies in schoolchildren population reported conflicting results. Song et al.24 showed in 54 Korean asthmatics (aged 6–15 years) with positive hyperresponsiveness challenge test (HCT) a significantly higher periostin levels than 29 healthy controls children (medians: 76ng/mL vs. 71ng/mL, p=0.017). Also, periostin levels were significantly correlated with HCT, peripheral blood eosinophils, and fraction of exhaled nitric oxide (FeNO). On the contrary, no correlation between periostin levels and lung function, eosinophil cationic protein, or total IgE levels was observed. The AUC for the periostin levels (cut-off 90ng/mL) for predicting asthma was 0.63 [0.52–0.74]. Also, Ionue et al.27 reported that serum periostin levels were significantly higher in 28 Japanese asthmatic children compared to 27 children without asthma (medians: 134ng/mL vs. 112ng/mL, p=0.012). The periostin levels correlated with peripheral eosinophil count (r=0.28, p=0.036), but not with FeNO. The AUC for periostin was 0.75. The authors remark as a limitation the fact that almost 90% of their asthmatics had allergic rhinitis. In another study of the same group28 on 304 elementary school-age children reported no increase in the serum periostin level in children with allergic diseases (e.g. rhinitis, atopic dermatitis, food allergy and asthma) compared with healthy children. No correlation was observed between the serum level of periostin and either peripheral blood eosinophilia or serum total IgE level.28 Moreover, a recent study on 551 Korean schoolchildren (∼12 years old) found that serum periostin levels are not associated with allergic rhinitis nor allergic sensitisation.29 In this regard, the fact that the present study uses the API algorithm that contains allergic rhinitis and eczema among their criteria for the classification of our study groups might produce obscure differences on periostin levels.
In an adult asthmatic population, the higher periostin serum levels correlated with specific diseases characteristics including older age at onset of asthma, higher blood eosinophil counts and lower pulmonary function.30 In addition, high periostin levels were associated with a higher prevalence of concomitant nasal disorders such as aspirin-intolerant asthma and chronic sinusitis with nasal polyps.30,31 A small, preliminary study reported that periostin deposition in the airway sub-epithelium of patients with asthma was associated with a decline in FEV1 over 20 years, and might correlate with collagen I deposition.32
In contrast, the relation of periostin serum levels and asthma severity and lung function in schoolchildren revealed different and conflict results. Konradsen et al.25 in 96 Swedish children (7–19 years of age) reported that periostin serum levels were similar among children with severe and moderate asthma; and periostin levels did not correlate with FeNO, blood eosinophils, or serum IgE. But lower FEV1% was found only in the group of children with high FeNO and high periostin. However, recently, a study on 50 Spanish children (6–13 years old) with poorly-controlled asthma reported no correlation between serum periostin and FEV1, FeNO, total IgE levels or blood eosinophils.33
The present study also has several limitations. First, as a cross-sectional study on pre-schoolers with mild-moderate disease (∼3 wheezing episodes in the previous year) we cannot know if periostin could be different in children with more severe disease. Second, no control group (pre-schoolers without recurrent wheezing/asthma) were included in the study. Third, our study had a small number of participants. Fourth, unfortunately pulmonary function test and FeNO was not performed; even though they are not easy to perform in the pre-school population it would be important to establish if serum periostin level is related with lung function or FeNO.
In conclusion, in contrast to our hypothesis recurrent wheezing pre-schoolers with positive API (∼atopic or type 2 inflammation asthma) have no higher periostin serum levels than those with negative API. No correlation of serum periostin levels and peripheral blood eosinophils was found. More studies in pre-schoolers with a bigger sample size and control group need to be done to confirm this finding.
Ethical disclosuresConfidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
FundingDivision of Pediatrics, Pontificia Universidad Catolica de Chile (Grant # 5-14).
Conflicts of interestThe authors declare that there are no conflicts of interest.