Hymenoptera venom-allergic patients frequently present multiple sensitisations.
ObjectivesTo define the allergic profile by components in wasp allergic patients. To study the usefulness of specific IgE to components in cases of double sensitisation.
Materials and methodsWasp allergic patients who needed Polistes and/or Vespula venom immunotherapy were included. Before immunotherapy and after two years of treatment the following specific IgE (sIgE) levels were measured: Apis mellifera, Vespula spp. Polistes spp., rVes v 5, rPol d 5, nVes v 5, nPol d 5, nVes v 1, nPol d 1, nApi m 1, nApi m 2 and peroxidase. Skin tests with venoms were performed. Based on the sIgE and the skin test results, Polistes and/or Vespula immunotherapy was administered.
ResultsThirteen patients were included. Double sensitisation to Polistes/Vespula was detected in eight patients. Sensitisation to rVes v 5 and rPol d 5 was found in two of eight cases, to nVes v 1 and nPol d 1 in eight of 13 cases, and to nVes v 5 and nPol d 5 in 2 of 13 cases. Three patients received double immunotherapy with both wasps. One patient was treated with Vespula and nine with Polistes. sIgE levels decreased after two years of treatment. In patients who showed double sensitisation but were treated with only one venom, sIgE to both venoms decreased.
ConclusionsComponents analysis can be useful to study double positivity. In case of doubt, double immunotherapy should be administered. Phospholipase was found to be a major allergen in our population.
Patients with hymenoptera venom allergy frequently present multiple sensitisations, which can sometimes complicate the choice of immunotherapy (IT). Double sensitisation to honeybee and wasp venom has been detected in up to 59% of patients.1
This multiple positivity can have several causes: (a) true multiple sensitisation, (b) sensitivity due to cross-reactivity, or (c) sensitisation to cross-reactive carbohydrate determinants (CCDs).1,2
Cross-reactivity between honeybee and wasp venoms is weak. It may be due to the hyaluronidase components (Api m 2, Ves v 2 and Pol d 2), which present a 50% similarity and contain CCDs.3–5
The high level of cross-reactivity between venoms of the different species of wasps is explained by the similarity of the venoms and of the structure of their allergens.6 Cross-reactivity between wasps of the Vespinae family (Vespula, Dolichovespula and Vespa) and Polistes is generally lower than within the Vespinae family.4,7 The phospholipases of Vespula and Polistes (Ves v 1 and Pol d 1) are very similar, but there is less similarity between antigen 5 of each venom 5 (Ves v 5 and Pol d 5)3; for this reason, the Pol d 5 and Ves v 5 allergen components are considered to be markers of primary sensitisation to wasp venoms and, in particular, to Polistes and Vespula venoms.
There is a considerable volume of research into cross-reactivity between honeybee and wasp venoms,1,2,5,8–10 but this is not the case for Vespula and Polistes venoms.11,12 The present study was therefore undertaken with the following objectives: (a) to determine the molecular allergenic profile (by components) of a sample of patients allergic to the venom of wasps present in our region and (b) to investigate the usefulness of the determination of the serum levels of specific immunoglobulin E (sIgE) to the individual allergen components in cases of double positivity to wasp venoms.
Materials and methodsPatients with a diagnosis of allergic systemic reaction following wasp stings, who needed Polistes and/or Vespula venom immunotherapy, and attended in our outpatient section in 2010 were included in the study.
Skin prick tests (SPT) and intradermoreaction were performed before immunotherapy using Polistes spp., Vespula spp. and A. mellifera venom extracts (ALK-Abelló, Madrid, Spain). These tests were considered positive if a concentration ≤1μg/l produced a papule ≥3mm diameter with erythema in the skin prick test or a papule ≥5mm diameter with erythema in the intradermoreaction.
Before immunotherapy and after two years of treatment the following sIgE were determined: sIgE to complete extracts of honey bee (Apis mellifera), Vespula (Vespula spp.), Polistes (Polistes spp.) venom and recombinant allergens rVes v 5 and rPol d 5 analysed by ImmunoCAP (Phadia) and sIgE to the natural allergens nVes v 5, nPol d 5, nVes v 1, nPol d 1, nApi m 1 and nApi m 2, as well as to horseradish peroxidase as a marker for CCDs using the ADVIA Centaur® (ADVIA) system (Siemens Medical Solutions Diagnosis, Tarrytown, New York, USA). sIgE values greater than 0.35kU/l were considered positive.
Based on the sIgE levels and the results of the skin tests, immunotherapy (IT) was initiated with the corresponding venoms (P. dominulus and/or Vespula spp., Pharmalgen ALK-Abelló). When double IT was required, maintenance treatment was performed with the administration of one venom each month in an alternating regimen. The initiation phase of the IT was always performed in a cluster schedule.
All the patients were informed about the study and consented to take part in it.
Results13 patients (nine male and four female) aged between 8 and 52 years (mean age, 36.6 years) were included in the study. Based on the Müeller classification of systemic reactions, three patients suffered grade I reactions, six patients grade II, three patients grade III, and one patient grade IV.
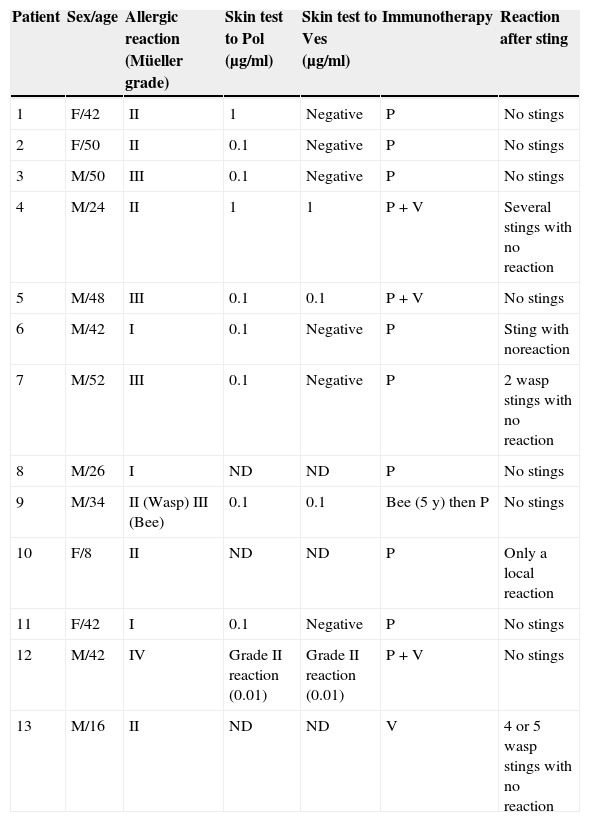
The clinical characteristics of the patients are summarised in Table 1.
Characteristics of the patients, skin tests, immunotherapy prescribed and reactions to stings.
| Patient | Sex/age | Allergic reaction (Müeller grade) | Skin test to Pol (μg/ml) | Skin test to Ves (μg/ml) | Immunotherapy | Reaction after sting |
|---|---|---|---|---|---|---|
| 1 | F/42 | II | 1 | Negative | P | No stings |
| 2 | F/50 | II | 0.1 | Negative | P | No stings |
| 3 | M/50 | III | 0.1 | Negative | P | No stings |
| 4 | M/24 | II | 1 | 1 | P+V | Several stings with no reaction |
| 5 | M/48 | III | 0.1 | 0.1 | P+V | No stings |
| 6 | M/42 | I | 0.1 | Negative | P | Sting with noreaction |
| 7 | M/52 | III | 0.1 | Negative | P | 2 wasp stings with no reaction |
| 8 | M/26 | I | ND | ND | P | No stings |
| 9 | M/34 | II (Wasp) III (Bee) | 0.1 | 0.1 | Bee (5 y) then P | No stings |
| 10 | F/8 | II | ND | ND | P | Only a local reaction |
| 11 | F/42 | I | 0.1 | Negative | P | No stings |
| 12 | M/42 | IV | Grade II reaction (0.01) | Grade II reaction (0.01) | P+V | No stings |
| 13 | M/16 | II | ND | ND | V | 4 or 5 wasp stings with no reaction |
Abbreviations: F, female; M, male; P, Polistes; V, Vespula; ND, not done.
These tests were performed in 10 patients (not carried out in patients 8, 10 and 13) and were positive in all 10. Double positivity (Vespula/Polistes) was detected in four cases (patients 4, 5, 9 and 12).
One patient (patient 12) developed systemic grade II reactions to Vespula and Polistes (0.01μg/ml in both cases) (Table 1).
Determination of sIgE (Table 2)sIgE to nApi m 1 and nApi m 2 (ADVIA) was positive only in patient 9, who had suffered a systemic reaction after a bee sting. This patient also had elevated levels of sIgE to Apis (Phadia).
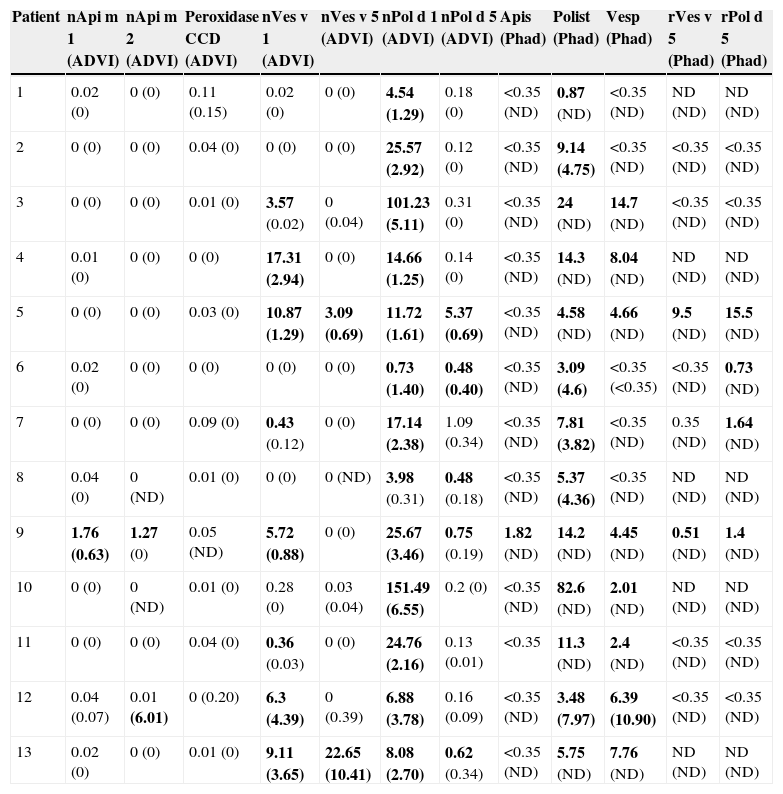
Specific IgE levels.
| Patient | nApi m 1 (ADVI) | nApi m 2 (ADVI) | Peroxidase CCD (ADVI) | nVes v 1 (ADVI) | nVes v 5 (ADVI) | nPol d 1 (ADVI) | nPol d 5 (ADVI) | Apis (Phad) | Polist (Phad) | Vesp (Phad) | rVes v 5 (Phad) | rPol d 5 (Phad) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.02 (0) | 0 (0) | 0.11 (0.15) | 0.02 (0) | 0 (0) | 4.54 (1.29) | 0.18 (0) | <0.35 (ND) | 0.87 (ND) | <0.35 (ND) | ND (ND) | ND (ND) |
| 2 | 0 (0) | 0 (0) | 0.04 (0) | 0 (0) | 0 (0) | 25.57 (2.92) | 0.12 (0) | <0.35 (ND) | 9.14 (4.75) | <0.35 (ND) | <0.35 (ND) | <0.35 (ND) |
| 3 | 0 (0) | 0 (0) | 0.01 (0) | 3.57 (0.02) | 0 (0.04) | 101.23 (5.11) | 0.31 (0) | <0.35 (ND) | 24 (ND) | 14.7 (ND) | <0.35 (ND) | <0.35 (ND) |
| 4 | 0.01 (0) | 0 (0) | 0 (0) | 17.31 (2.94) | 0 (0) | 14.66 (1.25) | 0.14 (0) | <0.35 (ND) | 14.3 (ND) | 8.04 (ND) | ND (ND) | ND (ND) |
| 5 | 0 (0) | 0 (0) | 0.03 (0) | 10.87 (1.29) | 3.09 (0.69) | 11.72 (1.61) | 5.37 (0.69) | <0.35 (ND) | 4.58 (ND) | 4.66 (ND) | 9.5 (ND) | 15.5 (ND) |
| 6 | 0.02 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0.73 (1.40) | 0.48 (0.40) | <0.35 (ND) | 3.09 (4.6) | <0.35 (<0.35) | <0.35 (ND) | 0.73 (ND) |
| 7 | 0 (0) | 0 (0) | 0.09 (0) | 0.43 (0.12) | 0 (0) | 17.14 (2.38) | 1.09 (0.34) | <0.35 (ND) | 7.81 (3.82) | <0.35 (ND) | 0.35 (ND) | 1.64 (ND) |
| 8 | 0.04 (0) | 0 (ND) | 0.01 (0) | 0 (0) | 0 (ND) | 3.98 (0.31) | 0.48 (0.18) | <0.35 (ND) | 5.37 (4.36) | <0.35 (ND) | ND (ND) | ND (ND) |
| 9 | 1.76 (0.63) | 1.27 (0) | 0.05 (ND) | 5.72 (0.88) | 0 (0) | 25.67 (3.46) | 0.75 (0.19) | 1.82 (ND) | 14.2 (ND) | 4.45 (ND) | 0.51 (ND) | 1.4 (ND) |
| 10 | 0 (0) | 0 (ND) | 0.01 (0) | 0.28 (0) | 0.03 (0.04) | 151.49 (6.55) | 0.2 (0) | <0.35 (ND) | 82.6 (ND) | 2.01 (ND) | ND (ND) | ND (ND) |
| 11 | 0 (0) | 0 (0) | 0.04 (0) | 0.36 (0.03) | 0 (0) | 24.76 (2.16) | 0.13 (0.01) | <0.35 | 11.3 (ND) | 2.4 (ND) | <0.35 (ND) | <0.35 (ND) |
| 12 | 0.04 (0.07) | 0.01 (6.01) | 0 (0.20) | 6.3 (4.39) | 0 (0.39) | 6.88 (3.78) | 0.16 (0.09) | <0.35 (ND) | 3.48 (7.97) | 6.39 (10.90) | <0.35 (ND) | <0.35 (ND) |
| 13 | 0.02 (0) | 0 (0) | 0.01 (0) | 9.11 (3.65) | 22.65 (10.41) | 8.08 (2.70) | 0.62 (0.34) | <0.35 (ND) | 5.75 (ND) | 7.76 (ND) | ND (ND) | ND (ND) |
Abbreviations: CCD, cross-reactive carbohydrate determinants; Polist, Polistes; Vesp, Vespula; ND, not done.
Brackets: Levels of sIgE after two years of immunotherapy.
In bold: high values of sIgE.
sIgE to horseradish peroxidase CCD was negative in all the patients.
sIgE to Vespula (Phadia) was positive in eight of the 13 patients but only two of these eight patients showed positive sIgE to rVes v 5 (Phadia) and a further two to nVes v 5 (ADVIA). However, sIgE to nVes v 1 (ADVIA) was elevated in eight of the 13 patients.
sIgE to Polistes (Phadia) was positive in all 13 patients. sIgE to rPol d 5 (Phadia) was elevated in 4 of 8 patients. sIgE to nPol d 5 (ADVIA) was positive in 5 of the 13 patients, and sIgE to nPol d 1 (ADVIA) was elevated in all 13 patients studied.
Double sensitisations evidenced by sIgE levels showed the following profiles: 8 of 13 cases (patients 3–5 and 9–13) were positive to Polistes and Vespula (Phadia); two of eight cases (patients 5 and 9) were positive to rVes v 5 and rPol d 5 (Phadia); eight of 13 cases (patients 3–5, 7, 9, 11–13) were positive to nVes v 1 and nPol d 1 (ADVIA) and two of 13 cases (patients 5 and 13) to nVes v 5 and nPol d 5 (ADVIA).
Immunotherapy and response of the sIgE levelsImmunotherapy (IT) was prescribed in all the patients. Three patients (patients 4, 5 and 12) received double IT with both wasp venoms. One patient (patient 9) had been previously treated with Apis immunotherapy for five years and begun Polistes immunotherapy. One patient was treated only with Vespula extract and eight only with Polistes.
After two years of IT, the sIgE levels decreased in all cases (Table 2). In patient 12, who received double IT, the sIgE levels to Polistes and to Vespula (Phadia) increased but the sIgE levels to nVes v 1 and to nPol d 1 (ADVIA) fell.
Patients treated with Polistes immunotherapy but who had elevated sIgE to Vespula (patients 3, 7, 9 and 11) also presented a decrease in the sIgE levels to Vespula. In one patient (patient 13) who received Vespula IT the sIgE levels to Polistes also fell.
No re-sting tests were performed. Five patients suffered stings while receiving the IT. Only one had a local reaction, the others had no reaction at all (Table 1).
DiscussionThe diagnostic study of patients who have presented allergic reactions to hymenoptera stings frequently reveals multiple sensitisations, which complicates the choice of the appropriate venom for IT.2,5,8–13 Several methods have been used to determine the responsible venom in these cases, including the study of sIgE to allergen components, the IgE-inhibition test, and the basophile activation test. It is currently agreed that determination of serum levels of sIgE to allergen components is the most useful test in patients with multiple sensitisation.1,2,5,8,9,12,13
All our patients presented sIgE to the complete venom of Polistes spp. (Phadia) and to nPol d 1 (ADVIA); Pol d 1 was therefore found to be a major allergen (100% of patients). However sIgE to Pol d 5 was only detected in half of the patients (Phadia, four of eight) or less than half (ADVIA, 5 of 13), making antigen 5 a minor antigen in our population.
Eight of the 13 patients had sIgE to Vespula (Phadia). Only two of these patients had sIgE to rVes v 5 (Phadia) and another two to nVes v 5 (ADVIA), meaning that Ves v 5 was also a minor antigen. Ves v 1 was positive in eight of 13 patients, indicating it to be a major allergen.
In the study published by the Hymenoptera Committee of the Spanish Society of Allergology and Clinical Immunology (SEAIC),12 elevated serum levels of sIgE to the recombinant allergen components rVes v 5 and rPol d 5 were detected in 52% of patients; however the majority of patients (21/25) had sIgE to the natural allergens nVes v 5 and nPol d 5. That group concluded that the diagnostic yield was higher when natural allergen components are used. In our study we found no differences between the recombinant (Phadia) and the natural (ADVIA) allergen components.
Müller et al.,1 in Switzerland, found that 96% of subjects allergic to Vespula had high levels of sIgE to rVes v 5 using the ADVIA system. In another study, those same authors found that study of the sensitivity to rVes v 5 (Phadia) was optimal (100% of patients sensitive only to Vespula), whereas only 33% of their patients presented sIgE to rVes v 1 (Phadia).2 However, Seismann et al.,5 in Germany, found a higher level of positivity to rVes v 1 than to rVes v 5.
Sensitisation to the allergen components of wasp venom varies depending on the geographic region studied, the predominant wasp in the region, and the extract used for the study (recombinant or natural).
In our study we detected double positivity (skin tests and/or sIgE) to Vespula/Polistes (Phadia) in eight patients. In four of these patients, the skin tests were positive with both wasp venoms (patients 4, 5, 9 and 12) and all four also showed double positivity to phospholipases; double positivity to antigen 5 was only observed in patient 5. Double IT was administered to three patients but in patient 9 it was decided to perform IT only with Polistes because the levels of sIgE to rPol d 1 and rPol d 5 were significantly higher than the levels to Vespula. In three of the remaining four patients, IT was performed with Polistes (patients 3, 10 and 11) and in patient 13 IT was performed with Vespula.
In the five patients with single positivity, the determination of sIgE to Vespula and Polistes (Phadia), with or without skin tests, was sufficient to establish the venom to be used for IT.
Thus, the medical history, the determination of sIgE to Polistes and Vespula (Phadia) and the skin tests with both wasp venoms would have been sufficient to reach a diagnosis in the majority of our patients and to determine the venom with which to perform vaccination. Study by allergen components would only be useful in case of doubt caused by double positivity with similar sIgE levels. We believe that when there is doubt about the responsible wasp, a good approach is to administer double IT.
In other published studies, the determination of sIgE to allergen components has also been found to be more useful than other techniques in cases of double positivity to hymenoptera, although the majority of those studies involved patients with Apis/Vespula sensitisation.2 The majority of cases of double positivity to honey bee/wasps are due to sensitisation to CCDs.3,5,13 Neis MM,9 in a study of patients sensitised to Apis and/or Vespula, obtained best results on measuring the levels of sIgE to allergen components in the patients with double positivity. Seismann et al.5 came to the same conclusion in their study, finding that the use of rVes v 1 and rVes v 5 identified the majority of subjects allergic to Vespula.
In cases of double sensitisation to wasps (Vespula/Polistes) Monsalve et al.12 measured sIgE to major natural allergen components (nVes v 1, nVes v 2, nVes v 5, nPol d 1 and nPol d 5); these measurements enabled them to demonstrate sensitisation to only a single wasp in 69% of the 45 cases—to Vespula in nine patients and to Polistes in 22. Double positivity was confirmed in 14 (31%) of cases. There are no other studies that demonstrate the usefulness of the measurement of sIgE to allergen components in cases of double positivity to Polistes/Vespula. In a study published by Caruso B et al.,11 45 patients with double positivity to Vespula and P. dominulus were studied by CAP and skin tests. IgE-inhibition tests were performed in all patients. In 25 patients P. dominulus inhibited Vespula vulgaris and those patients received IT with P. dominulus. In six patients V. vulgaris inhibited P. dominulus and those patients were vaccinated with V. vulgaris. The results did not show differences between the venoms in the remaining 14 patients, and those patients received double IT.
In our study, the choice of the IT would appear to have been correct in all patients, as those patients who suffered stings thereafter (five patients) presented only a local reaction or no reaction and, after two years of IT, the levels of sIgE had fallen in all cases. However, in patient 12, who received double IT, the levels of sIgE to Polistes and to Vespula (Phadia) were higher after two years of IT, although the levels of sIgE to nVes v 1 and to nPol d 1 (ADVIA) had fallen. In patients vaccinated with P. dominulus but who had elevated sIgE to Vespula (patients 3, 7, 9, 11), the levels of sIgE to Vespula also fell. In one patient (patient 13) who was vaccinated with Vespula, the levels of sIgE to Polistes also fell at two years.
Conclusions- (1)
Our population was found to be positive mainly to Polistes, the dominant insect in our region.
- (2)
Phospholipase was found to be a major allergen component whereas antigen 5 was a minor allergen component.
- (3)
In cases of double positivity to both Polistes and Vespula spp. based on the determination of sIgE to complete venoms (Phadia) and/or positive skin tests, the allergic status of the patients can be better defined by measuring sIgE to allergen components.
- (4)
In case of doubt, double immunotherapy should be administered.
The authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Protection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals for this investigation.
Financial supportNo external financial support was provided for the study.
Conflict of interestAgustín Galán Nieto works in the R&D department of ALK-Abelló, manufacturer of hymenoptera venom immunotherapy. The other authors have no conflicts of interest to declare.
The authors thank nurses of our sections, Elena Andrés, Ma José Muñoz, Ma José Ruiz and Mª Dolores Díaz, for their collaboration.
The cases described were presented at Sesiones Interhospitalarias de la Sociedad Madrid-Castilla La Mancha de Alergología e Inmunología Clinica, February 2013, Madrid, Spain.