Immunoglobulin replacement therapy is an effective route of management for both infections and non-infectious complications in predominantly antibody deficiency (PAD). Trace levels of IgA (ranged from 0.4 to 2500mg/ml), which exist in all immunoglobulin products, could lead to an increased susceptibility for adverse reactions in PAD patients. Furthermore, the exact mechanism which stimulates the anti-IgA antibody production in PAD is still unknown. The aim of this study was to evaluate IgG anti-IgA antibodies in PAD patients receiving intravenous immunoglobulin (IVIg) and its predisposing factors.
MethodsAvailable patients with confirmed diagnosis of PAD, who underwent regular IVIg replacement therapy in our centre, were enrolled in the study. Control group included 24 healthy individuals as the negative control and eight symptomatic patients with IgA deficiency as the positive control groups. IgG anti-IgA antibodies level was measured by the ELISA method.
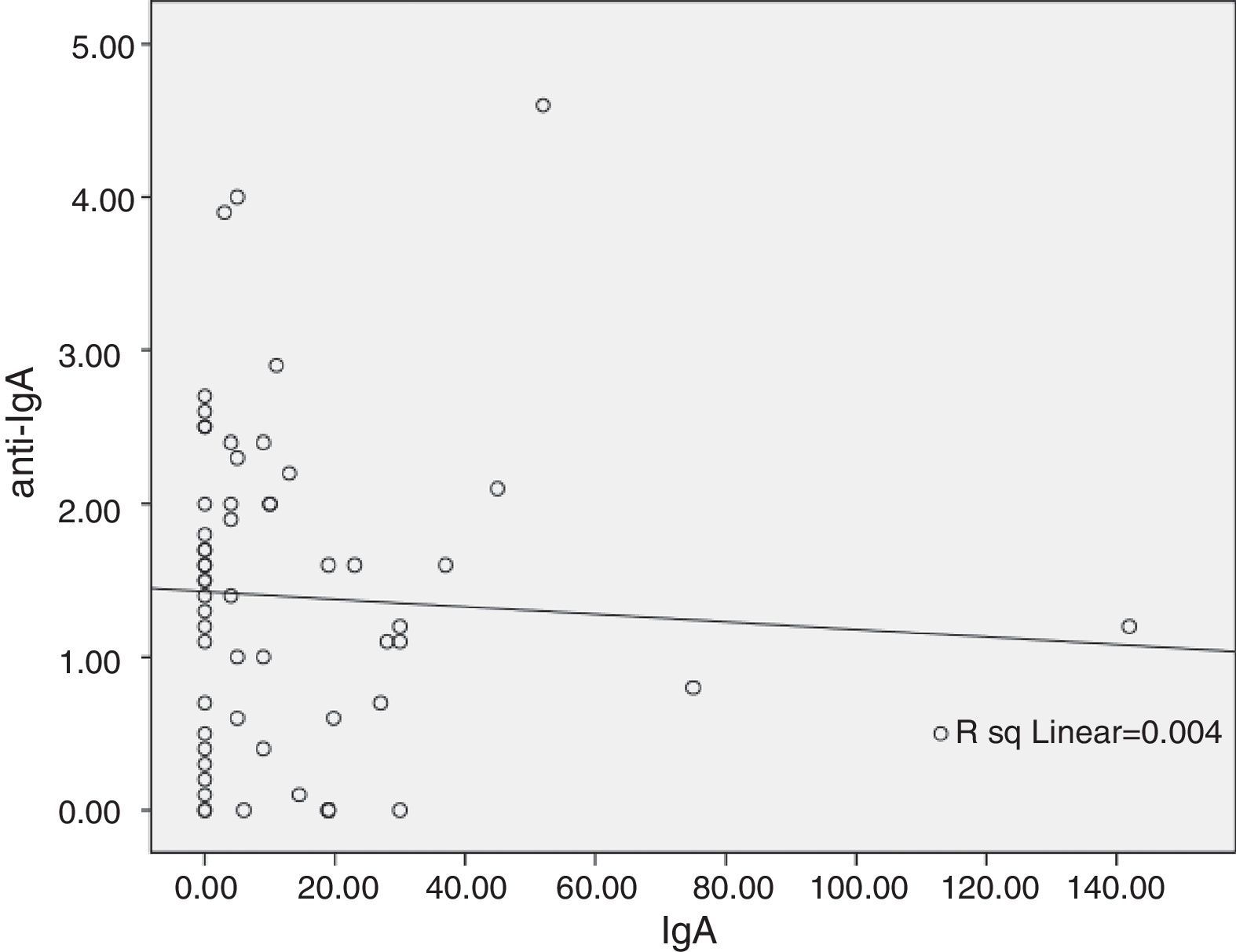
ResultsA significant difference was observed between Anti-IgA level of common variable immunodeficiency (CVID) and other PAD groups (p=0.02). Moreover, six CVID patients were seropositive for the IgG anti-IgA antibody, with higher susceptibility to the adverse reactions (p<0.001). IgG anti-IgA level has a negative relationship with serum IgA level (r=−0.06) and IVIg treatment duration (r=−0.006).
ConclusionOur data suggested that there was a significant association between anti-IgA antibody presence and the adverse reactions, especially in CVID patients with higher susceptibility to produce this constitutional antibody.
Predominantly antibody deficiencies (PAD) are the most common types of primary immunodeficiency disorders. Patients with PAD have increased proneness to recurrent infections and consequent morbidity and mortality due to defect in humoral immunity.1 Intravenous immunoglobulin (IVIg) is the mainstay treatment of different types of PAD such as common variable immunodeficiency (CVID), X-linked agammaglobulinaemia (XLA), and hyper IgM (HIgM) syndrome. These disorders present with hypogammaglobulinaemia especially with very low or absent serum IgA level. The major component of IVIg is IgG, however, IVIg contains trace amounts of IgM and IgA, which may be recognised as unfamiliar antigens in PAD patients.2–7 Anti-IgA antibodies were detected in up to 40% of patients with selective IgA deficiency (SIgAD) and 10–25% of patients with CVID.8–11
The use of immunoglobulin preparations may lead to mild to severe adverse reactions.12,13 The adverse reactions to this agent have been attributed to the serum IgG anti-IgA antibodies.14 The role of these antibodies in causing adverse reactions has not been clearly understood. Some of the factors which could lead to an adverse reaction due to anti-IgA antibodies, include anti-IgA serum concentration, anti-IgA isotype (IgG anti-IgA or IgE anti-IgA), and anti-IgA specificity, as well as the route of immunoglobulin administration (intramuscular, intravenous, or subcutaneous), baseline serum IgA level and IgA content of the immunoglobulin product.15
Adverse reactions have also been reported in many patients with undetectable levels of these antibodies. On the other hand, many patients with high levels of these antibodies might not encounter any adverse reaction.14 Hence, the correlation between IgG anti-IgA antibodies and adverse reactions to IVIg is still controversial.
This study was designed to evaluate the characteristics of IgG anti-IgA antibodies and related adverse reactions following IVIg administration in PAD patients. Furthermore, we evaluated the predisposing factors on IgG anti-IgA antibody levels, such as serum IgA level, duration of treatment and presence of autoimmunity.
Patients and methodsPatientsSixty-seven patients with confirmed diagnosis of PAD, who were under regular IVIg replacement therapy at the children's Medical Center hospital, were enrolled in this study. The diagnosis was made according to the diagnostic criteria of the European Society for Immunodeficiencies (ESID) and the Pan American Group for Immunodeficiency (PAGID).16 All patients were treated with the same manufacturer IVIg (Intratect 10%, the only available product in Iran). According to the manufacturer's guideline, this preparation contains 96% IgG and its maximum IgA content is 2000μ/ml.
Patients received 400–500mg/kg IVIg therapy every 3–4 weeks during their follow-up period. All clinical, immunological and para-clinical data of studied patients were recorded in a prepared questionnaire. Five millilitre Ethylene Diamine Tetraacetic Acid (EDTA) blood samples were collected from the patients just before the regular infusion of immunoglobulin preparation. The control groups consisted of 24 healthy individuals (negative control) and eight symptomatic SIgAD patients (positive control). The process of this study was approved by the Ethics Committee of the Tehran University of Medical Sciences and informed consent was obtained from all subjects or their parents.
Detection of IgG anti-IgA antibodiesIgG Anti-IgA antibodies were detected by Enzyme-linked immunosorbent assay (ELISA) method. Briefly, 96-well microplates (Biovendor, Czech Republic) were coated with 100μl of diluted serum samples and incubated at 37°C for 1h. Secondly, plates were washed three times with wash solution. Thirdly, 100μl of conjugate solution was added into each well and the wells incubated at room temperature for 1h. The wells were washed with wash solution three times and plates incubated at room temperature for 10min after adding 100μl of substrate solution. In order to stop the blue colour development, 100μl of stop solution was added. The absorbance of each well was determined at 450nm by using a microplate reader.
Statistical analysisStatistical analysis was done using SPSS software package version 16.0. One-sample Kolmogrov–Smirnov test was used to estimate the distribution type of the data. Parametric and non-parametric analyses were performed based on the finding of this evaluation. A p value of 0.05 or less was considered statistically significant. Moreover anti-IgA seropositive cut off point was generated based on the healthy controls (negative controls) group. In this manner, PAD subjects were considered seropositive if the value of IgG anti-IgA antibodies was equal to or larger than the two standard deviations values in the healthy control groups. Binary logistic regression was used in order to analyse the impact of different parameters on the adverse reaction occurrence, especially the anti-IgA level effect.
ResultsA total of sixty-seven PAD patients (11 males and 56 females), including 34 patients with CVID, 23 patients with XLA, and 10 patients with HIgM syndrome with a mean diagnosis age of 8.3±7.7 years were enrolled in this study. Patients were followed for 508 patient-years with a mean follow-up period of 8.1±5.3 years per patient over a 30-year period of time. Thirty-six patients (54%) were the result of a consanguineous marriage. History of autoimmunity was recorded in 20 patients (29.8%, Table 1). During the follow-up period, mild adverse reactions were documented in 13 (19.4%) patients and none of the patients suffered from moderate or severe side effects. The most common feature was chills which was recorded in 12 cases, followed by fever (9 cases), feeling of cold (8 cases), backache (8 cases) and headache (6 cases).
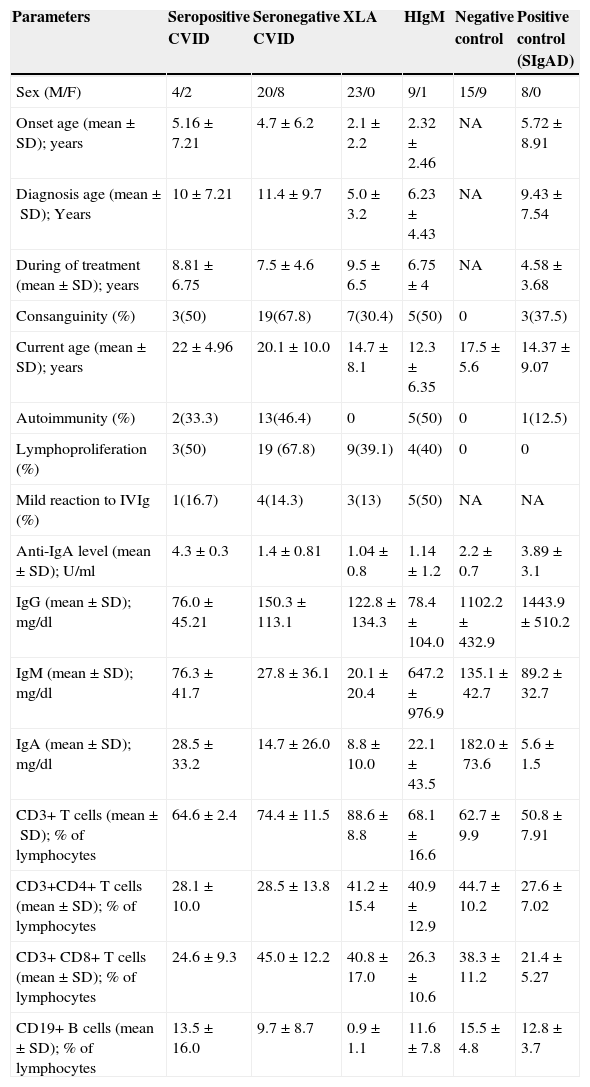
Demographic and immunological data of 67 PAD patients receiving IVIg in comparison with 24 healthy controls and 8 IgA deficient patients as positive controls.
| Parameters | Seropositive CVID | Seronegative CVID | XLA | HIgM | Negative control | Positive control (SIgAD) |
|---|---|---|---|---|---|---|
| Sex (M/F) | 4/2 | 20/8 | 23/0 | 9/1 | 15/9 | 8/0 |
| Onset age (mean±SD); years | 5.16±7.21 | 4.7±6.2 | 2.1±2.2 | 2.32±2.46 | NA | 5.72±8.91 |
| Diagnosis age (mean±SD); Years | 10±7.21 | 11.4±9.7 | 5.0±3.2 | 6.23±4.43 | NA | 9.43±7.54 |
| During of treatment (mean±SD); years | 8.81±6.75 | 7.5±4.6 | 9.5±6.5 | 6.75±4 | NA | 4.58±3.68 |
| Consanguinity (%) | 3(50) | 19(67.8) | 7(30.4) | 5(50) | 0 | 3(37.5) |
| Current age (mean±SD); years | 22±4.96 | 20.1±10.0 | 14.7±8.1 | 12.3±6.35 | 17.5±5.6 | 14.37±9.07 |
| Autoimmunity (%) | 2(33.3) | 13(46.4) | 0 | 5(50) | 0 | 1(12.5) |
| Lymphoproliferation (%) | 3(50) | 19 (67.8) | 9(39.1) | 4(40) | 0 | 0 |
| Mild reaction to IVIg (%) | 1(16.7) | 4(14.3) | 3(13) | 5(50) | NA | NA |
| Anti-IgA level (mean±SD); U/ml | 4.3±0.3 | 1.4±0.81 | 1.04±0.8 | 1.14±1.2 | 2.2±0.7 | 3.89±3.1 |
| IgG (mean±SD); mg/dl | 76.0±45.21 | 150.3±113.1 | 122.8±134.3 | 78.4±104.0 | 1102.2±432.9 | 1443.9±510.2 |
| IgM (mean±SD); mg/dl | 76.3±41.7 | 27.8±36.1 | 20.1±20.4 | 647.2±976.9 | 135.1±42.7 | 89.2±32.7 |
| IgA (mean±SD); mg/dl | 28.5±33.2 | 14.7±26.0 | 8.8±10.0 | 22.1±43.5 | 182.0±73.6 | 5.6±1.5 |
| CD3+ T cells (mean±SD); % of lymphocytes | 64.6±2.4 | 74.4±11.5 | 88.6±8.8 | 68.1±16.6 | 62.7±9.9 | 50.8±7.91 |
| CD3+CD4+ T cells (mean±SD); % of lymphocytes | 28.1±10.0 | 28.5±13.8 | 41.2±15.4 | 40.9±12.9 | 44.7±10.2 | 27.6±7.02 |
| CD3+ CD8+ T cells (mean±SD); % of lymphocytes | 24.6±9.3 | 45.0±12.2 | 40.8±17.0 | 26.3±10.6 | 38.3±11.2 | 21.4±5.27 |
| CD19+ B cells (mean±SD); % of lymphocytes | 13.5±16.0 | 9.7±8.7 | 0.9±1.1 | 11.6±7.8 | 15.5±4.8 | 12.8±3.7 |
CVID: Common variable immunodeficiency, HIgM: hyper IgM syndrome, NA: not applicable, SIgAD: selective IgA deficiency, Seropositive: level of anti-IgA>3.6U/ml, Seronegative: level of anti-IgA<3.6U/ml, XLA: X-linked agammaglobulinaemia.
Anti-IgA was detectable in 88% (n=59) of patients including 33 CVID (97%), 18 XLA (78%) and eight HIgM patients (80%). Mean of anti-IgA antibody levels was 1.6±1.0U/ml in PAD patients receiving IVIg, 2.2±0.7U/ml in negative healthy controls and 3.8±3.1U/ml in positive controls, including SIgAD patients.
The association between the level of anti-IgA antibody and the type of PAD was analysed by one-way ANOVA test. A significant difference was seen between CVID and XLA groups (1.7±1.2 vs. 1.04±0.81U/ml; F=4.58, p=0.02). Comparison of the anti-IgA antibody level between XLA group and positive control group, which consists of eight SIgAD patients, also showed a significant difference (p=0.001).
However, there was no significant relationship between anti-IgA antibody level and the presence of autoimmunity in the case group (1.5±1.1 vs. 4.0±1.5U/ml; p=0.21). CVID patients with autoimmunity (3.5±2.3U/ml) had a higher anti-IgA antibody level compared to other CVID patients (1.8±1.2U/ml), but this difference was not significant (p=0.1). Similarly, the level of the anti-IgA antibody in HIgM patients with autoimmunity was higher compared to CVID and XLA patients (0.6±0.7 vs. 1.6±1.4U/ml; p=0.26).
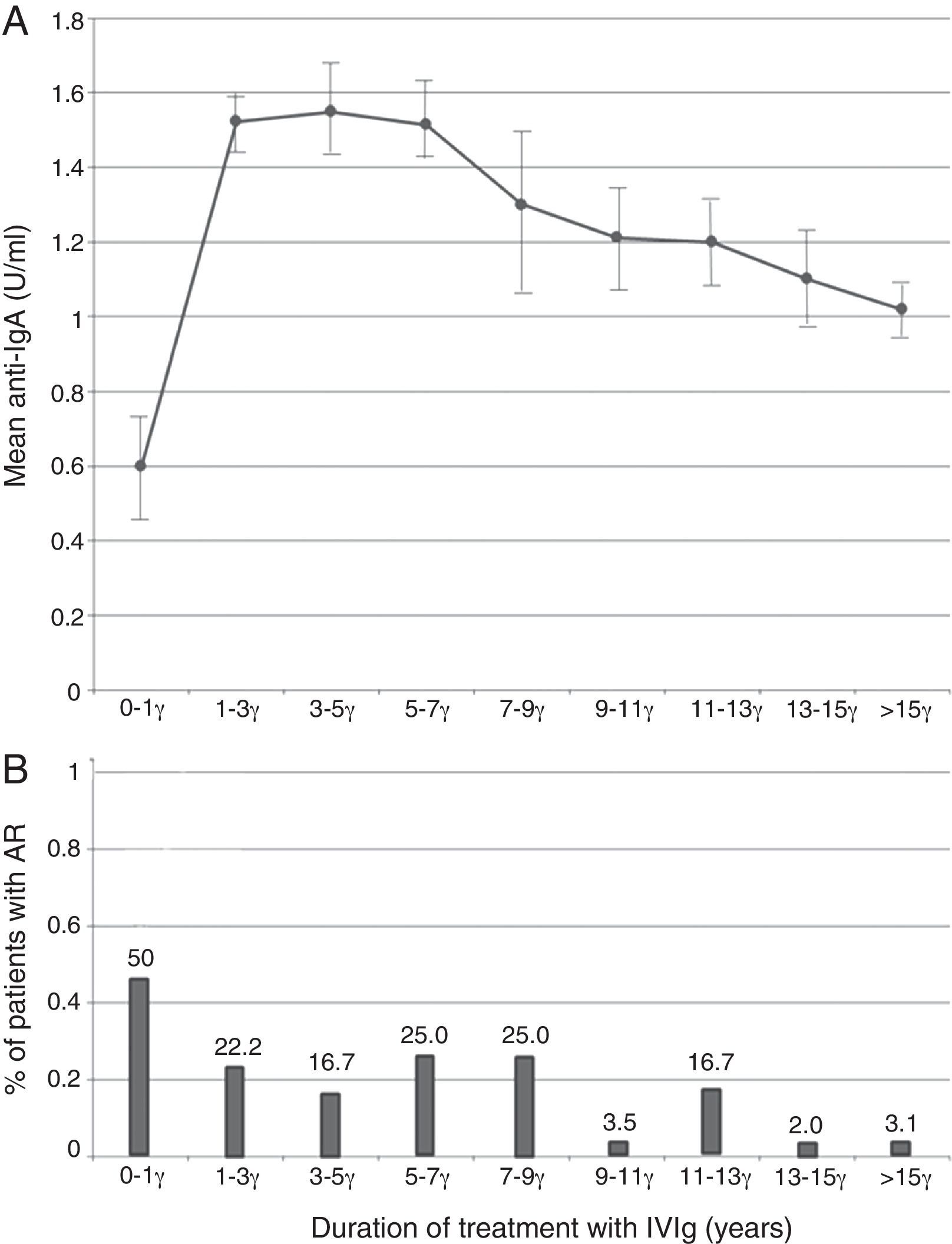
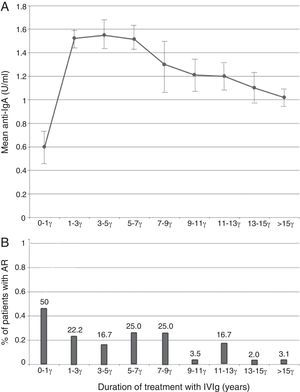
The probable association between anti-IgA antibody level and baseline IgA level concentration at the time of diagnosis was analysed by linear regression model. A negative relationship was seen (r=−0.06, Fig. 1), however, this relationship was not statistically significant (p=0.64). Analysis of the correlation between anti-IgA antibody level and IVIg treatment duration showed an inverse relationship (r=−0.006, p=0.96), and anti-IgA antibody peak level was observed during the first year of IVIg administration (Fig. 2A). Surprisingly, the number of patients with an adverse reaction followed a similar pattern of anti-IgA antibody level during the IVIg treatment duration (r=−0.13, p=0.29). The peak of adverse reaction occurred during the first year of treatment, in which 50% of patients had a positive record of adverse reaction (Fig. 2B). Multivariable analysis for different parameters of mild reactions occurrence was conducted by the logistic regression model. Presence of autoimmunity (OR=0.007, CI=0.001–0.01) and duration of treatment (OR=0.80, CI=0.66–0.98) correlated indirectly with adverse reaction manifestation. As a result there was no significant association between anti-IgA antibody level and adverse reactions (OR=1.02, CI=0.04–3.52); this variable was excluded from the model.
Seropositive patientsAccording to the cut-off point (3.6U/ml) based on the healthy controls anti-IgA antibody (2.2±0.7U/ml); only six PAD patients (8.9%), all with CVID, were seropositive for IgG anti-IgA antibody. All of these six patients had IgA levels less than 6.0mg/dl at the diagnosis time. Autoimmunity and lymphoproliferation were observed in two (33.3%) and three (50%) patients of the anti-IgA seropositive group, respectively. In seronegative CVID patients, autoimmunity was detected in 46.2%, as well as, lymphoproliferation in 69.2%. However, these differences were not statistically significant (p=0.48 and p=0.07, respectively). Mild adverse reactions were reported in 16.7% (n=1) of seropositive and 14.3% (n=4) of seronegative patients. The frequency of adverse reactions differed significantly between two groups (p<0.001). The difference of anti-IgA antibody level between CVID patients with complete IgA deficiency and those with partial IgA deficit was not statistically significant (p value=0.38).
DiscussionIgG anti-IgA antibodies was linked with adverse reactions in SIgAD patients under IVIg therapy in previous studies.9,12,17 Although the detection probability of a class-specific anti-IgA antibody in this group is only 17–32%, immunoglobulin product consumption is forbidden as a preventive treatment for SIgAD.18–22 Five to 7% of asymptomatic SIgAD patients also presented a subclass-specific anti-IgA antibody.23,24 Sufficient evidence to clarify the correlation between serum IgG anti-IgA antibody and adverse effects in other PID patients under IVIg treatment is not available. However, these antibodies seem to have a role in some mild adverse reactions such as headache, nausea, and malaise.25 As no patient in this survey had encountered severe adverse reactions such as anaphylaxis, we could not evaluate the association of IgG anti-IgA antibodies with severe adverse reactions. Nevertheless, the result of this study showed a significantly higher frequency of the mild adverse effects in CVID patients with elevated serum IgG anti-IgA antibody. In spite of that, the same investigation was not proven in all PAD patients. Previously, some studies discussed the condition of a group of patients with anti-IgA antibodies, who could tolerate IVIg adverse reactions.10,26 On the other hand, several factors are associated with adverse reaction in PAD patients including dosage of immunoglobulin, rate of infusion, pre-medication, and the presence of infection. These factors are responsible for the lack of a definite conclusion about the role of the anti-IgA antibody.27,28
Nadorp et al. documented that occurrence of adverse symptoms was directly related to higher concentrations of anti-IgA antibody. They also suggested that patients with IgG anti-IgA antibody demonstrated increased catabolism of IgA.29 Efforts for making threshold level for this phenomenon, however, failed because of different methods and values across studies.15
Surprisingly, the IgG anti-IgA value in healthy normal controls was higher than the mean level in the PAD patients in this study. Although previous literatures documented 0.5–6% seropositivity in healthy individuals,20,24 which could be increased to 59% if the threshold was considered, more or less suggesting anti-IgA as a natural antibody.30 In contrast, reduction of the natural antibodies such as iso-haemagglutinin, heterophilic, antierythrocytic, and antibacterial was proved in patients with PAD.31–33 Therefore, XLA and HIgM patients, with a class switched antibody production defect, comparing to the healthy individuals, failed to show anti-IgA antibody.
In contrast, selected groups of CVID patients with more developed humoral immunity are able to produce this antibody, as well as many other natural or auto antibodies.
In previous studies, it was suggested that there is a great tendency for seropositivity in adults,15 however this study on paediatric PAD rolls out this hypothesis. Indeed, declined serum IgA levels might be a prerequisite of anti-IgA antibody production.8,9 Our results were consistent with previous studies, because all of the seropositive patients had IgA levels below 0.06g/l. Furthermore, there was a negative relationship between anti-IgA antibody level and serum IgA level. So, screening for IgG anti-IgA antibodies in PAD patients with low serum IgA, who are supposed to receive IVIg, is more reasonable.
Some studies reported that IgG anti-IgA antibody levels decreased gradually over continuous therapy either with IVIg.9,12,26,34–37 According to our results, there was a negative correlation between anti-IgA antibody level and duration of treatment. Patients, who received IVIg for a longer period of time, had a lower anti-IgA level. This phenomenon could be the result of an induced tolerance against IgA, as a new antigen with peripheral regulatory T cells, or other anergy mechanisms.38
Based on a previous study, the IgA quantity in the infusion product may also have a role in such a reaction. Most of the patients with adverse reactions have been treated by the products containing IgA levels of 50mg/ml or greater.15 As a result of using the same type of IgA and a same route of administration in our study, we could not evaluate the effect of the IgA content and the route of administration on the adverse reactions and the anti-IgA antibody level. However, some studies suggested that subcutaneous infusions might be well tolerated by patients with anti-IgA antibodies and decrease the likelihood of adverse reactions. The latter statement supports a hypothesis, which proposed that the tolerance induction is due to the gradual release of an antigen into the immune system.15,39 This is due to some reports revealing that products with low IgA have been successfully tried in some patients who had repeated reaction to products with high IgA.8,16
We tried for the first time to investigate the anti-IgA antibodies in a paediatric population with early onset of the PAD disease. In comparison to the other reports, the mean diagnosis age of our case population was lower. This might be due to the higher rate of parental consanguinity in Iran.40
In conclusion, our study suggested that long term treatment with IVIg causes a gradual decline in the IgG anti-IgA level. Moreover, a low serum level of IgA is a predisposing factor for the IgG anti-IgA antibody production, especially, in paediatric patients. Screening for IgG anti-IgA antibody is reasonable in patients with low serum IgA prior to the IVIg treatment. We also suggested that there is an association between the IgG anti-IgA antibodies presence and the adverse reactions in CVID patients. However, larger multicentre prospective studies are required to illuminate the accurate role of these antibodies and assess their association with severe adverse reactions.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the responsible clinical research ethics committee and in accordance with those of the world medical association and the Helsinki declaration.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Conflict of interestThe authors have no conflict of interest to declare.