Low vitamin D status is linked to increased incidence of food allergy and intestinal inflammation. Whether vitamin D status is associated with immunological changes in children with gastrointestinal food allergy (GFA) remains unclear.
MethodsForty-nine GFA children (aged 2–11 years old) were enrolled in this study. Serum 25-hydroxyvitamin D (25OHD) level, total immunoglobulin E (IgE), specific IgE against allergens, circulating regulatory T lymphocytes (Tregs), and blood eosinophil numbers were measured.
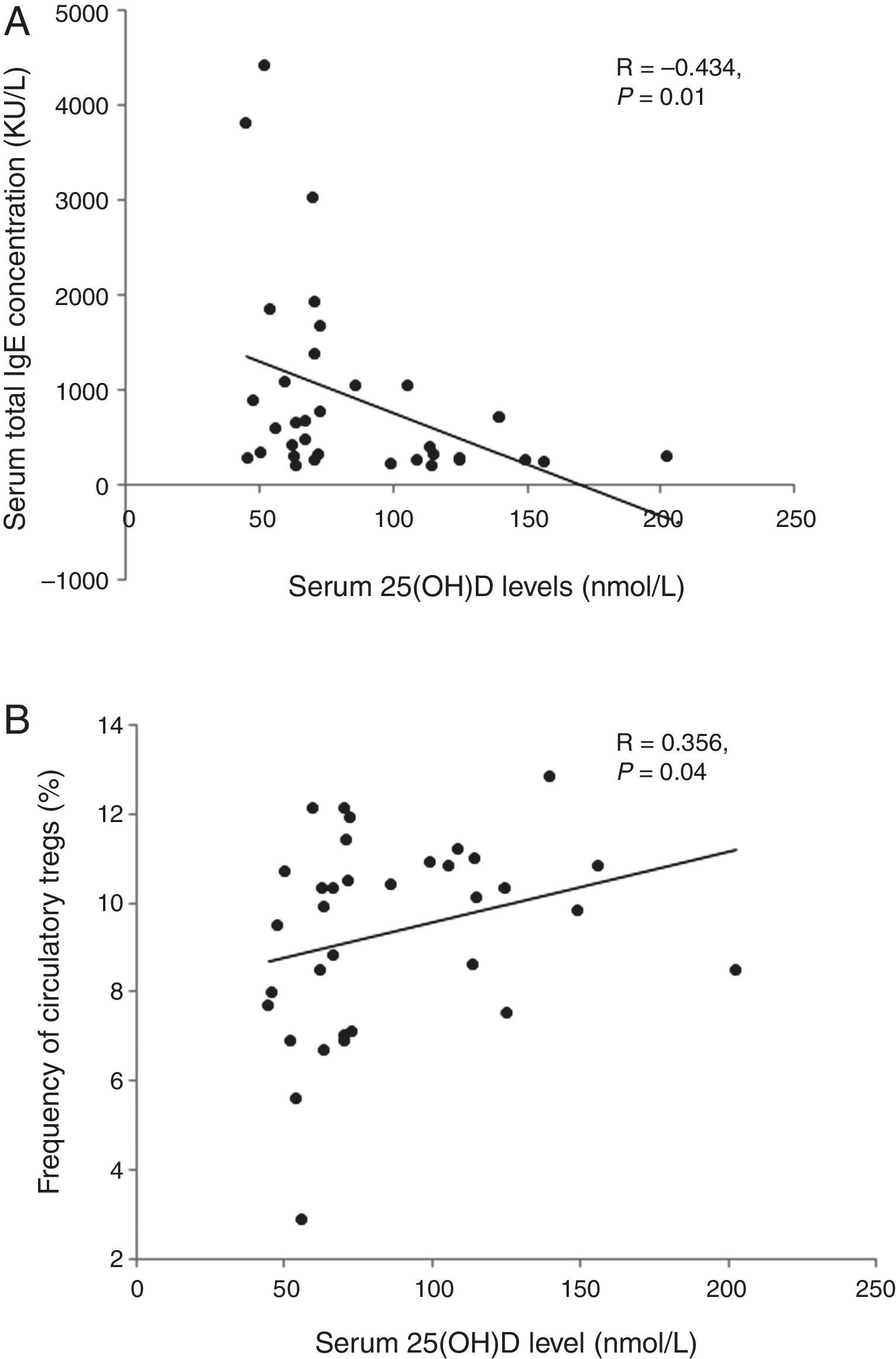
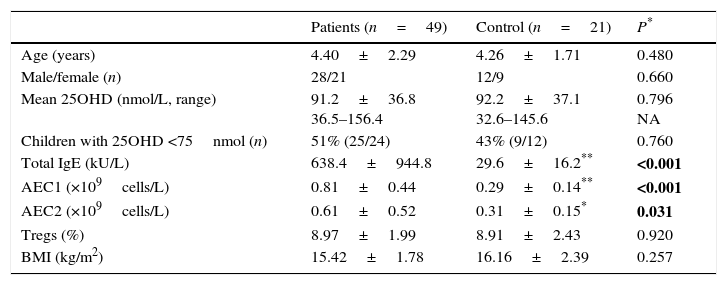
ResultsLevels of serum 25OHD in the GFA children ranged 35.5–156.4nmol/L, with a mean value similar to that of the healthy controls. Compared to those with normal 25OHD (≥75nmol/L), GFA children with low 25OHD (<75nmol/L) had increased total IgE (84% vs. 54%, P<0.05), persistent blood eosinophilia (56% vs. 25%, P<0.05), and delayed resolution of symptoms after food allergen elimination (odds ratio 3.51, 95% CI 1.00–12.36, P<0.05). Among the GFA children with elevated total IgE, those with low 25OHD had lower circulatory Tregs (8.79±2.4% vs. 10.21±1.37%, P<0.05), higher total IgE (1197.5±1209.8 vs. 418.5±304.6kU/L, P<0.05), and persistent eosinophilia (0.61±0.52 vs. 0.31±0.15×109cells/L, P<0.05) compared to those with normal 25OHD. In addition, serum 25OHD concentrations inversely correlated with total IgE (R=−0.434, P<0.05), and positively with Treg population (R=0.356, P<0.05).
ConclusionLow serum vitamin D status correlates with stronger allergic immune response in GFA children.
Gastrointestinal food allergy (GFA), an adverse immune-mediated reaction to food proteins, is the major cause for digestive system dysfunctions in children and is linked to multiple gastroenteropathies such as eosinophilic oesophagitis and gastroenteritis, irritable bowel syndrome, and inflammatory bowel diseases.1 The prevalence of food allergy has been increasing among children living in both developed and developing countries with dramatic impacts on the quality of life of affected children, and financial burdens on the families and social health care systems. There are no proactive treatments available for food allergy. Consequently, the mainstay of therapy is the choice of avoidance.2 Although risk factors and aetiology for food allergy still remain unknown, multiple factors may trigger the escalating trend in the past decade. Studies have revealed that vitamin D may be the important modifiable environmental and social factor for food allergy.3
Vitamin D deficiency and insufficiency is common among children and infants.4,5 Vitamin D is a sterol hormone with well-established function for regulating mineral and bone homeostasis. Mounting evidence supports vitamin D associates with allergic immune responses by regulating the functions of immune cells such as dendritic cells, T and B lymphocytes, mast cells,6–9 with an overall immunosuppressive effect on allergens-triggered hypersensitive immune reaction. Vitamin D and vitamin D receptor (VDR)-mediated signalling also involve in the proliferation of intestinal mucosal epithelial cells and maintaining intact intestinal barrier. Low serum vitamin D level or decreased intestinal VDR signalling can cause impaired intestinal barrier integrity and increase exposure to pathogens from intestinal lumen, and predispose to more-severe and persistent intestinal injury in murine colitis.10–12 The increased permeability and persistent inflammation in gut contribute to the pathological process of GFA.1
Several observational studies suggested that low vitamin D status is associated with the occurrence or the pathological process of food allergy. Low serum vitamin D in pre-neonatal, neonate, or pregnant women was related to increased incidence rate of childhood food allergy or sensitisation,13,14 and infants with challenge-proven food allergy as well as food-sensitised infants with severe atopic dermatitis have low serum vitamin D.15,16 How serum vitamin D status correlates with immunological changes in children who are affected by GFA is less investigated. In this study, we determined serum vitamin D status and its relationship with immunological and clinical outcomes in children with symptomatic GFA.
Materials and methodsSample collection and ethics statementBlood samples were collected from 49 children aged 2–11 years old with confirmed diagnosis of GFA during the period from April 2014 to July 2016 at Gastroenterology Clinic of Nanjing Children's Hospital (Nanjing, Jiangsu), a general hospital for children's health care and diseases. The Gastroenterology Department at NCH has 50 beds for in-patient care. GFA was initially diagnosed based on medical history, clinical symptoms, physical examination, negative research for other causes of the gastrointestinal symptoms including gastrointestinal infections and lactose intolerance. The diagnosis was confirmed by patients’ response to therapy of dietary allergen elimination (milk, eggs, seafood, soybean, peanut and other tree nuts, and wheat, or the known offending food allergen) and positive response to reintroduction of food allergens after the resolution of gastrointestinal symptoms or a history of recurrent gastrointestinal symptoms upon the offending food allergen reintroduction. Children with any previous history of medical/surgical problems or taking any medication were excluded. Resolution of gastrointestinal symptoms and peripheral blood eosinophil numbers before and after dietary avoidance were recorded. Blood samples from 21children aged 2–10 years old were used as age-matched controls.
The Clinical Research Ethics Committee of Nanjing Children's Hospital approved this study (approval number: 201412001-1). Parents of eligible children were asked to provide written informed consent. The study was in accordance with the code of ethics of the World Medical Association (Declaration of Helsinki).
25-hydroxyvitamin D quantification by enzyme immunoassaySerum 25OHD levels were determined using an EIA Kit (IDS, Boldon, UK) since 25OHD in circulation is considered the best biomarker of vitamin D metabolic status and represents vitamin D from all sources.17
Analysis of circulating populations of CD4+ lymphocytes and regulatory T cellsWe used the method described by Hardy et al. to detect proportion of circulatory CD4+ lymphocytes and Tregs.18 Briefly, whole blood (100μl) was incubated for 30min at room temperature with 20μl of a staining cocktail (Cat. No. 560249, BD Biosciences, CA, USA) that contains FITC anti-human CD4 antibody, PE-Cy7 anti-human CD25 antibody and Alexa Fluor-647 anti-human CD127 antibody. Subsequently, erythrocytes were removed by adding BD FACS red blood cell lysing buffer (Cat. No. 349202, BD Biosciences, CA, USA). The samples were analysed on a BD FACS ARIA II with FACS express software.
Measurement of serum allergic parametersTotal IgE and sIgE for allergens against a panel of inhalant (dog, cat, horse, birch, timothy grass, mugwort, Dermatophagoides pteronyssinus (dust mite), and food allergens (milk, egg, soybean, cod, peanut, wheat flour) were measured using ImmunoCAP Specific IgE Blood Test (Pharmacia Diagnostics, Freiburg, Germany). Children with sIgE values ≥0.35kU/L were used to define allergic sensitisation. Total IgE concentration >166.3kU/L, the upper normal limit of the lab's IgE healthy reference (13–166.3kU/L), was classified as “increased”. Blood eosinophils were enumerated using a Coulter STKS counter (Beckman Coulter, Miami, FL, USA), and were expressed as absolute eosinophil counts. Blood eosinophilia is defined when eosinophil count is higher than 0.5×109cells/L.19
Statistical analysisDescriptive data are presented as mean±SD for numeric variables and in percentages or numbers for categorical variables. Categorical variables were compared by performing Fisher's exact test. Comparisons of numeric variables between two groups were performed with unpaired or paired samples t-test. Spearman or Pearson correlation test was used for comparisons between numerical variables depending on distribution of the variables. Additionally, multivariable logistic regression was used to assess whether the variables may be related to the odds ratio (OR) of gastrointestinal symptoms resolution after food allergens avoidance. All statistical analyses were performed by using SPSS software (v19, SPSS Inc., Chicago, IL, USA), and two-tailed P values of <0.05 were considered with statistical significance.
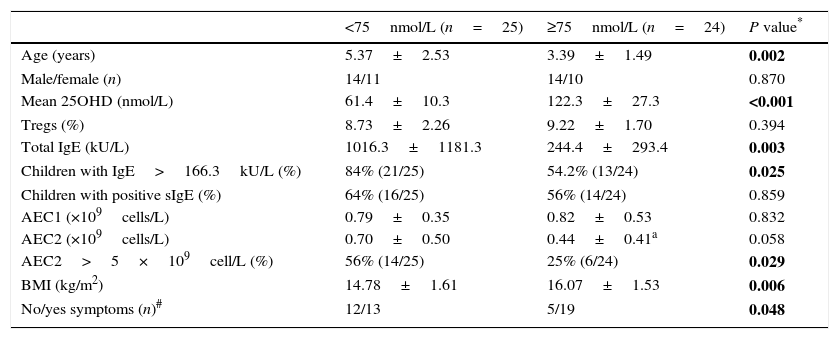
ResultsForty-nine GFA children and 21 age-matched healthy children were included in this study. There was no significant difference of age, gender, and body mass index (BMI) between these two groups (Table 1). Serum 25OHD levels in the GFA children ranged from 36.5 to 156.4nmol/L with a mean value of 91.2nmol/L. For comparison, serum 25OHD levels in age-matched healthy controls were 92.2nmol/L (ranged from 32.6 to 145.6nmol/L). However, low 25OHD (<75nmol/L, or 30ng/mL) were common in both GFA and control groups (Table 1). In general, GFA children had elevated total IgE and/or blood eosinophil numbers.
Characteristics of GFA children and control group.
| Patients (n=49) | Control (n=21) | P* | |
|---|---|---|---|
| Age (years) | 4.40±2.29 | 4.26±1.71 | 0.480 |
| Male/female (n) | 28/21 | 12/9 | 0.660 |
| Mean 25OHD (nmol/L, range) | 91.2±36.8 36.5–156.4 | 92.2±37.1 32.6–145.6 | 0.796 NA |
| Children with 25OHD <75nmol (n) | 51% (25/24) | 43% (9/12) | 0.760 |
| Total IgE (kU/L) | 638.4±944.8 | 29.6±16.2** | <0.001 |
| AEC1 (×109cells/L) | 0.81±0.44 | 0.29±0.14** | <0.001 |
| AEC2 (×109cells/L) | 0.61±0.52 | 0.31±0.15* | 0.031 |
| Tregs (%) | 8.97±1.99 | 8.91±2.43 | 0.920 |
| BMI (kg/m2) | 15.42±1.78 | 16.16±2.39 | 0.257 |
Abbreviations: AEC1/2, peripheral absolute eosinophil counts before (AEC1) and after (AEC2) one-week food-allergen avoidance; BMI, body mass index; Tregs, regulatory T cells; NA, not applicable.
Except for gender, values shown are mean±SD.
P value for parameter comparison between the GFA and the control groups. Categorical data were compared by the Fisher exact test. Numeric numbers were compared by unpaired t tests. Statistically significant differences are indicated in bold. P<0.05 were considered significant difference.
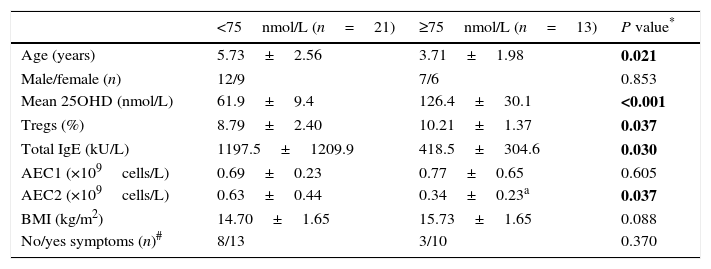
Serum concentration of 25OHD in the GFA children varied widely. The mean 25OHD level in the children with low 25OHD was significantly lower than those with normal 25OHD levels (≥75nmol/L) (Table 2). We noticed that GFA children with low 25OHD level had increased total IgE (IgE>166.3kU/L) and persistent blood eosinophilia after one-week food-allergen avoidance, while the eosinophil numbers in those with normal 25OHD rapidly decreased to normal range. The GFA children showed improvement and disappearance of gastrointestinal symptoms upon allergen avoidance (Table 2), but those with low 25OHD had delayed symptomatic resolution compared to those with normal 25OHD level (OR=3.51, 95% CI 1.00–12.36, P=0.048). Additionally, the GFA children with low 25OHD were older and had lower BMI than the 25OHD normal group. There was no difference in circulatory Treg subpopulation in GFA children with low and normal 25OHD. The age and BMI had no effects on total IgE, Tregs, and eosinophil numbers.
Comparisons of children with GFA based on serum 25OHD.
| <75nmol/L (n=25) | ≥75nmol/L (n=24) | P value* | |
|---|---|---|---|
| Age (years) | 5.37±2.53 | 3.39±1.49 | 0.002 |
| Male/female (n) | 14/11 | 14/10 | 0.870 |
| Mean 25OHD (nmol/L) | 61.4±10.3 | 122.3±27.3 | <0.001 |
| Tregs (%) | 8.73±2.26 | 9.22±1.70 | 0.394 |
| Total IgE (kU/L) | 1016.3±1181.3 | 244.4±293.4 | 0.003 |
| Children with IgE>166.3kU/L (%) | 84% (21/25) | 54.2% (13/24) | 0.025 |
| Children with positive sIgE (%) | 64% (16/25) | 56% (14/24) | 0.859 |
| AEC1 (×109cells/L) | 0.79±0.35 | 0.82±0.53 | 0.832 |
| AEC2 (×109cells/L) | 0.70±0.50 | 0.44±0.41a | 0.058 |
| AEC2>5×109cell/L (%) | 56% (14/25) | 25% (6/24) | 0.029 |
| BMI (kg/m2) | 14.78±1.61 | 16.07±1.53 | 0.006 |
| No/yes symptoms (n)# | 12/13 | 5/19 | 0.048 |
Abbreviations: AEC1/2, peripheral absolute eosinophil counts before (AEC1) and after (AEC2) one-week food-allergen avoidance; BMI, body mass index.
Values shown are mean±SD and number or frequency.
P value for parameter comparison between the GFA with low and normal 25OHD groups. Categorical data were compared using Fisher exact test while paired and unpaired t tests for numeric data. Statistically significant differences are indicated in bold. P<0.05 were considered with significant difference.
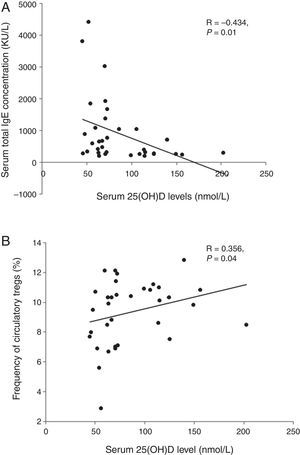
We analysed the associations between 25OHD and immunological characteristics of GFA children with elevated total IgE. As shown in Table 3, children with low 25OHD had higher total IgE, lower circulatory Tregs, and persistent blood eosinophilia (Table 3). Furthermore, bivariate relationship analysis showed serum 25OHD concentration correlated inversely with total IgE (Fig. 1A), but positively with circulatory Treg population (Fig. 1B).
Comparisons of GFA children with increased total IgE based on serum 25OHD.
| <75nmol/L (n=21) | ≥75nmol/L (n=13) | P value* | |
|---|---|---|---|
| Age (years) | 5.73±2.56 | 3.71±1.98 | 0.021 |
| Male/female (n) | 12/9 | 7/6 | 0.853 |
| Mean 25OHD (nmol/L) | 61.9±9.4 | 126.4±30.1 | <0.001 |
| Tregs (%) | 8.79±2.40 | 10.21±1.37 | 0.037 |
| Total IgE (kU/L) | 1197.5±1209.9 | 418.5±304.6 | 0.030 |
| AEC1 (×109cells/L) | 0.69±0.23 | 0.77±0.65 | 0.605 |
| AEC2 (×109cells/L) | 0.63±0.44 | 0.34±0.23a | 0.037 |
| BMI (kg/m2) | 14.70±1.65 | 15.73±1.65 | 0.088 |
| No/yes symptoms (n)# | 8/13 | 3/10 | 0.370 |
Abbreviations: AEC1/2, peripheral absolute eosinophil count before and after one-week food-allergen elimination; BMI, body mass index.
Values shown are mean±SD and number or frequency.
P value for parameter comparison between the low and normal 25OHD groups of the GFA children with elevated total IgE. Categorical data were compared using Fisher exact test while paired and unpaired t tests for numeric data. Statistically significant differences are indicated in bold. P<0.05 were considered with significant difference.
Correlations between serum 25OHD and total IgE and circulatory Tregs in gastrointestinal food allergic children (GFA) with elevated IgE. (A) Serum 25OHD concentration has an inverse correlation with total IgE. (B) Serum 25OHD concentration has a positive correlation with circulatory Treg cell population.
The prevalence of vitamin D insufficiency is very common.4,20,21 Low vitamin D status has been linked to a variety of disorders. We showed here that GFA children who have low serum vitamin D tend to have higher total IgE and persistent eosinophilia after withdrawal of the offending allergens. IgE and eosinophils have essential roles in type I hypersensitivity, which manifests in various allergic diseases, including food allergies. It was observed that improving vitamin D status in pregnancy or early infancy reduces the development of allergic disease in high-risk infants by inhibiting cytokine profiles associated with allergy.22 In mice, vitamin D deficiency causes IgE elevation and eosinophilia in neonatal allergic disease.23 Vitamin D plays a role in the regulation of immuno-surveillance and maintenance of immunological homeostasis in the gut through IgE-mediated hypersensitive immune responses, including inhibition of IgE production,7 reduction of release of cytotoxic granules by eosinophils,24 and eosinophils migration.25 Therefore, low vitamin D status may adversely affect gut immunological homeostasis in children suffering from GFA.
Another aspect of vitamin D on gut allergic immune response correlates with its capacity to promote the development or to enhance the activity of Tregs, a CD4+ T lymphocytes subgroup that is specialised for immune suppression in normal immune system and is crucial in controlling immune responses as well as maintaining the homeostasis of the immune system.26 The induction of Tregs is a key event in tolerance to food allergens.27 We found that the Treg population was lower in the low vitamin D group. Vitamin D can promote T lymphocyte to Treg transition and induces the development of Tregs via inducing an immature state of tolerogenic dendritic cells, the major cells for presenting antigens to T cells, by inhibiting their differentiation and maturation.28 Vitamin D status positively correlates with regulatory T cell function.9 In pregnant women, vitamin D deficiency impairs Treg cell function and elevates IgE receptor-expressing B cells.29 It is therefore of significance in monitoring vitamin D status of children with digestive system disorders including GFA.
In summary, our study provides novel information in that it is one of very few studies investigating whether lower vitamin D correlates with higher hypersensitive immune response in GFA children. Although the numbers were small, we were able to demonstrate that delayed resolution of eosinophilia and gastrointestinal symptoms was associated with low vitamin D status. Therefore, vitamin D normalisation could be beneficial for children who suffer from GFA.
Ethical disclosuresConfidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Protection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Source of fundingThe study was funded by a grant from Nanjing Municipal Department of Science and Technology (ZKX130391 to GY).
Conflict of interestThe authors declare no financial or other issues that might lead to conflict of interests. The author for correspondence is in possession of related documents of this study. All authors read and approved the manuscript.
The authors acknowledge all the patients and their families in the Pediatric Gastroenterology Unit, Nanjing Children's Hospital for their cooperation during the study period, and Dr. Y. Fan of Nanjing University Medical School for assistance with statistics.