Cow's milk protein allergy (CMPA) affects between 0.6 and 0.9% of the general population, and its treatment implies the total elimination of the intake of this protein. Camel's milk has been suggested as an alternative for patients over one year of age who suffer from CMPA due to the difference in the amino acid sequence from that of cow's milk. The objective of this study was to evaluate the safety and tolerability of camel's milk in children with CMPA.
MethodsCrossed clinical trial for the use of camel's milk vs. amino acid formula, carried out at the Dr. Federico Gómez Children's Hospital of Mexico (HIMFG) on patients between one and 18 years of age with diagnosed CMPA confirmed through double-blind, placebo-controlled food challenges (DBPCFCs). Only those whose allergies were confirmed were randomly placed into two groups: those to be administered camel's milk and those to be administered the amino-acid formula for two weeks, followed by a six-week wash-out period, and then a group crossing for a further two weeks.
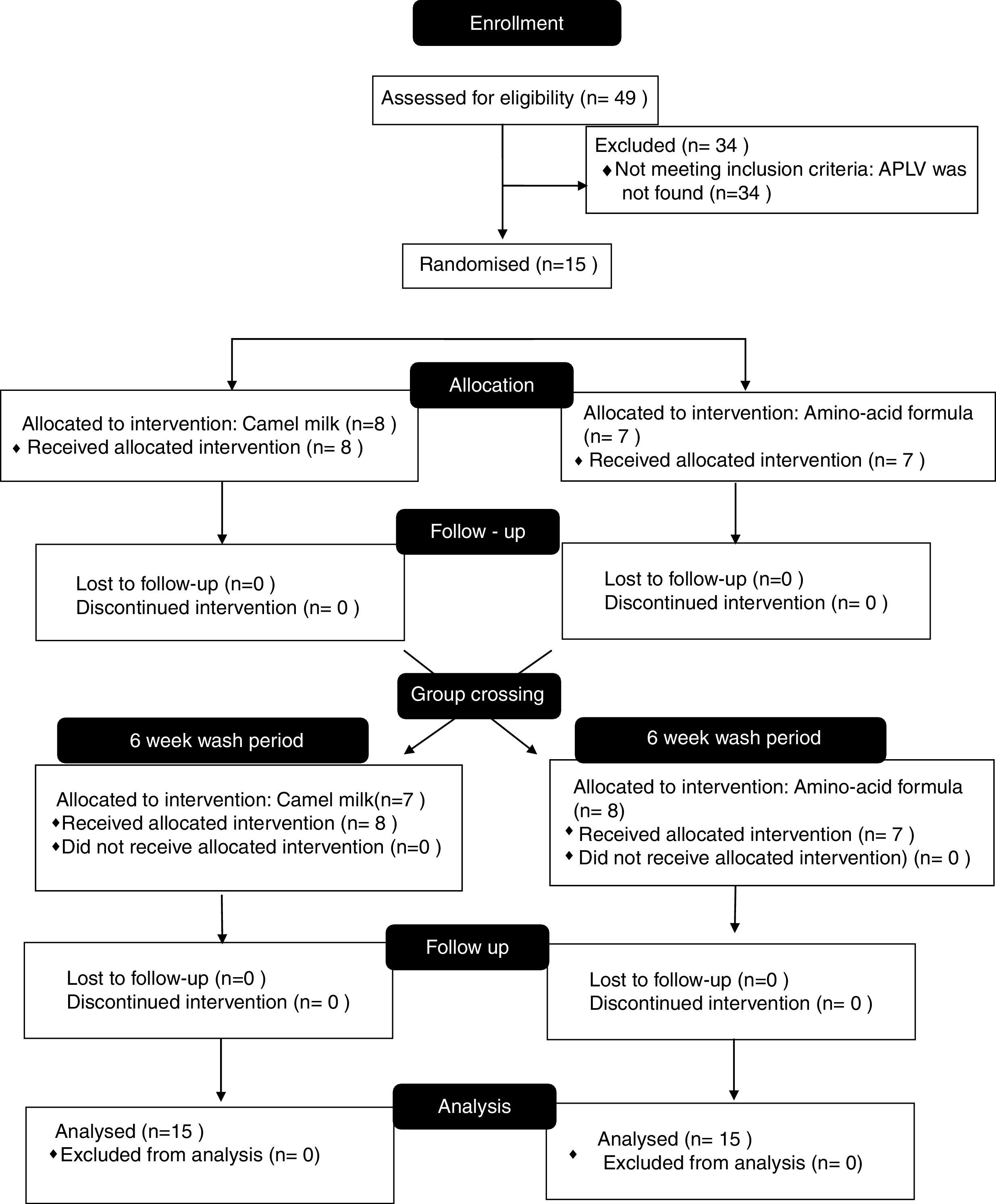
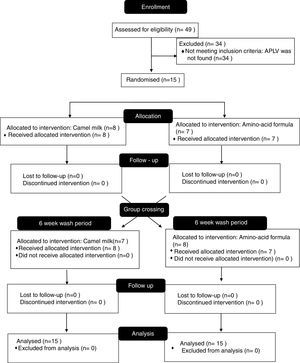
Results49 patients with suspected CMPA were included in the study; the diagnosis was confirmed through DBPCFCs in 15 patients, who were those who participated in the study. After having been administered camel's milk, none of the patients presented adverse effects.
Conclusions and clinical relevanceCamel's milk is safe and tolerable in patients above one year of age with CMPA and can be considered as a good alternative given the benefit of its taste compared to other formulas.
Cow's milk protein allergy (CMPA) affects between 0.6 and 0.9% of the general population, and is a public health issue that mainly affects the paediatric population.1,2 Although the diagnosis of CMPA is not simple, we know that double-blind, placebo-controlled food challenges (DBPCFCs) continue to be the gold standard for its diagnosis, especially in research studies.2–4 Current treatment for CMPA today implies totally eliminating the intake of the protein, which in most cases represents a huge challenge for the family and attending physicians.5–7
Whenever prescribing a diet free from cow's milk protein, it must be nutritionally adequate, which is why the objective is to reach a protein–calorie balance with an adequate amino-acid composition, as well as a proper source of calcium. It is necessary to complement the diet of children under the age of one with an extensively hydrolysed or amino-acid formula,8 however, low organoleptic acceptance, understood as the sensory perception of the physical qualities of matter, greatly limits the use of these formulas, as does adherence; furthermore, it has been noted that the smaller the amount of administered protein, the worse the flavour.9
After the first year of age, introducing solid foods into the baby's diet avoids the need for cow's milk protein.8 Formulas that are extensively hydrolysed with casein and whey, as well as amino acids, are designed for children under the age of one, acting as a substitute for cow's milk protein; after this first year of life, this protein is no longer necessary for their diet.8 However, it has been proven that children over the age of one who have restrictions regarding this product and its derivatives, absorb less protein and calories over all, even when aided by nutritionists.10 Cow's milk is highly consumed not only in Mexico, but around the world. According to the Mexican Secretariat of Economy and the General Directorate for Basic Industries, in 2010, 97 litres of cow's milk were consumed per inhabitant per year in Mexico,11 without taking into account that a significant amount of processed food contains cow's milk protein as a base ingredient.12,13
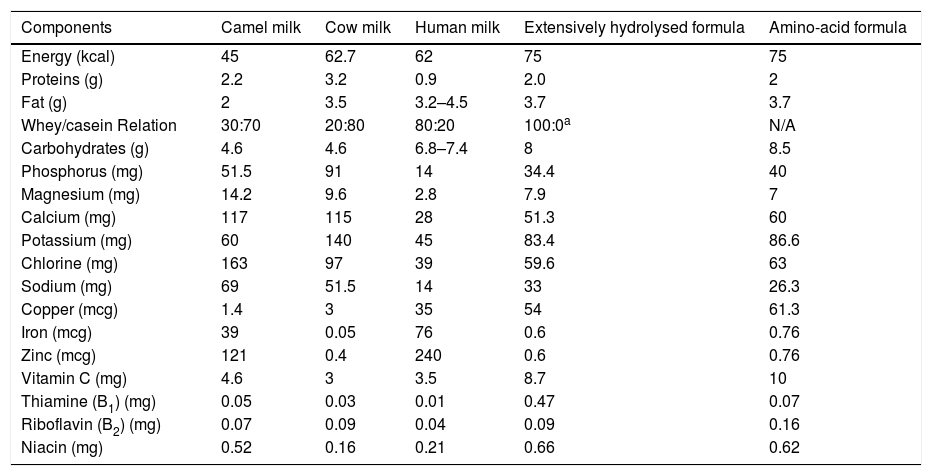
Camel's milk has been proposed as an alternative for patients who suffer from CMPA because of its different amino-acid sequence; furthermore, it presents low quantities of β casein (Bos d8) and no β lactoglobulins (Bos d5),14 and is adequately nutritionally balanced with essential components such as lactoferrin, immunoglobulins, lysozyme, and vitamin C15 (Table 1).
Nutritional composition of 100ml of camel milk and other milk formulas.25–27
| Components | Camel milk | Cow milk | Human milk | Extensively hydrolysed formula | Amino-acid formula |
|---|---|---|---|---|---|
| Energy (kcal) | 45 | 62.7 | 62 | 75 | 75 |
| Proteins (g) | 2.2 | 3.2 | 0.9 | 2.0 | 2 |
| Fat (g) | 2 | 3.5 | 3.2–4.5 | 3.7 | 3.7 |
| Whey/casein Relation | 30:70 | 20:80 | 80:20 | 100:0a | N/A |
| Carbohydrates (g) | 4.6 | 4.6 | 6.8–7.4 | 8 | 8.5 |
| Phosphorus (mg) | 51.5 | 91 | 14 | 34.4 | 40 |
| Magnesium (mg) | 14.2 | 9.6 | 2.8 | 7.9 | 7 |
| Calcium (mg) | 117 | 115 | 28 | 51.3 | 60 |
| Potassium (mg) | 60 | 140 | 45 | 83.4 | 86.6 |
| Chlorine (mg) | 163 | 97 | 39 | 59.6 | 63 |
| Sodium (mg) | 69 | 51.5 | 14 | 33 | 26.3 |
| Copper (mcg) | 1.4 | 3 | 35 | 54 | 61.3 |
| Iron (mcg) | 39 | 0.05 | 76 | 0.6 | 0.76 |
| Zinc (mcg) | 121 | 0.4 | 240 | 0.6 | 0.76 |
| Vitamin C (mg) | 4.6 | 3 | 3.5 | 8.7 | 10 |
| Thiamine (B1) (mg) | 0.05 | 0.03 | 0.01 | 0.47 | 0.07 |
| Riboflavin (B2) (mg) | 0.07 | 0.09 | 0.04 | 0.09 | 0.16 |
| Niacin (mg) | 0.52 | 0.16 | 0.21 | 0.66 | 0.62 |
Various authors have tried to test the tolerability of camel's milk on patients who are allergic to cow's milk protein, and have obtained favourable results; however, it is worth considering that most of these studies evaluate the tolerability of camel's milk in open trials, which could skew the results.
The objective of our study was to evaluate the safety of administering camel's milk to paediatric patients over the age of one with CMPA.
Materials and methodsA cross-over clinical trial was carried out at the Dr. Federico Gómez Children's Hospital of Mexico (HIMFG), from January to December 2016, to compare the safety between administering camel's milk vs. amino-acid formula on children diagnosed with CMPA. Patients from 1 to 18 years of age with suspected CMPA – understood as the presence of respiratory, cutaneous, gastrointestinal or nasal symptoms associated with the intake of cow's milk protein – were included in the initial study. Patients with allergies from other food sources were excluded from the study, as well as those under the treatment of immunosuppressive drugs, systemic steroids, antihistamines and anti-leukotrienes. None of the patients suffered from any concomitant chronic illnesses, except from rhinitis or asthma; notwithstanding, adequate symptom control was carried out when participating in the trial.
This trial was approved by the Ethics and Biosafety investigation committees at HIMFG (protocol number HIM/2016/006).
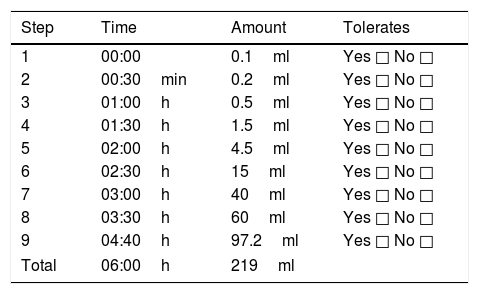
All patients, with prior informed consent, underwent a full medical evaluation by a certified paediatrician, including medical records and a physical examination. The first step was to confirm the diagnosis of CMPA by carrying out a DBPCFC with the cow's milk protein; in accordance with international guidelines on the matter,23 patients underwent a strict cow's milk protein elimination diet over six weeks with previous supervision and education regarding food that could contain the ingredient and label reading. At the end of these six weeks, a placebo or lactose-free milk was administered on two separate days, with dosage levels progressing every 30min, until reaching a volume of 240ml. The presence of abdominal, cutaneous, respiratory and nasal symptoms was monitored, and results were considered positive when objective symptoms were present, or subjective symptoms in three consecutive doses, and worsened or persisted after 40min.24
Having confirmed the diagnosis of CMPA, medical advice was newly given concerning the elimination diet and patients were summoned six weeks later. During this six-week period, patients were randomised by a random numbers generator which balanced out segments of five patients in one of two groups: one to be administered camel's milk and the other an amino-acid formula in accordance with Table 2. Neither the doctors in charge of administering and monitoring, nor the patients nor family members knew what the trial was about. Patient monitoring was carried out according to the times specified in Table 2 at the hospital, in case of the presence of abdominal, cutaneous, respiratory and nasal symptoms. Results were deemed positive when objective symptoms were present, or subjective symptoms present in three consecutive doses, and worsened or persisted after 40min.
Steps for administration of double-blind placebo-controlled oral challenge.
| Step | Time | Amount | Tolerates |
|---|---|---|---|
| 1 | 00:00 | 0.1ml | Yes □ No □ |
| 2 | 00:30min | 0.2ml | Yes □ No □ |
| 3 | 01:00h | 0.5ml | Yes □ No □ |
| 4 | 01:30h | 1.5ml | Yes □ No □ |
| 5 | 02:00h | 4.5ml | Yes □ No □ |
| 6 | 02:30h | 15ml | Yes □ No □ |
| 7 | 03:00h | 40ml | Yes □ No □ |
| 8 | 03:30h | 60ml | Yes □ No □ |
| 9 | 04:40h | 97.2ml | Yes □ No □ |
| Total | 06:00h | 219ml | |
Verum preparation (camel milk): amino-acid formula, camel milk (2.62g protein), sugar+vanilla flavouring.
Placebo preparation (amino-acid formula): amino-acid formula, sugar, vanilla flavouring.
In case of negative results throughout this period of hospital monitoring, patients would be released and given enough formula/milk so as to ingest 200ml per day over seven days. At the end of the intervention, an intermediate six-week cleansing period was given, in which neither intervention (formula or milk) was administered. The groups were then switched, so as to carry out the same procedure with the other intervention. Daily follow-up was given over the phone during these two weeks, and at the end of the whole process a physical examination was carried out by a paediatric allergist.
ResultsA total of 49 patients with suspected CMPA were evaluated, 49.0% male (95%CI 35.5–62.5) and 51.0% female (95%CI 37.4–64.4); at the time of trial inclusion, the mean age was 4.3 (1–13 years old). The most frequently mentioned symptoms related to cow's milk protein consumption were abdominal and mainly abdominal pain (Table 3).
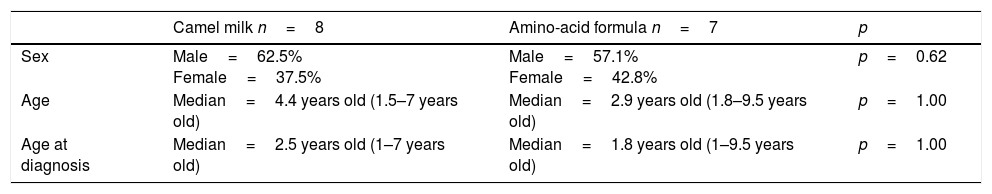
Description of the population included in the study in groups: camel milk and amino-acid formula.
| Camel milk n=8 | Amino-acid formula n=7 | p | |
|---|---|---|---|
| Sex | Male=62.5% Female=37.5% | Male=57.1% Female=42.8% | p=0.62 |
| Age | Median=4.4 years old (1.5–7 years old) | Median=2.9 years old (1.8–9.5 years old) | p=1.00 |
| Age at diagnosis | Median=2.5 years old (1–7 years old) | Median=1.8 years old (1–9.5 years old) | p=1.00 |
A DBPCFC for cow's milk protein was carried out on the 49 trial patients, confirming CMPA diagnosis in one third of the total (15 patients), nine males and six females with a mean age of four (1–9 years old) (Table 3).
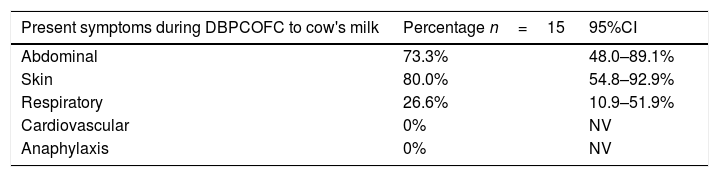
The most frequent symptoms in patients with confirmed CMPA were cutaneous, then abdominal (Table 4).
Symptoms presented in patients with DBPCFC positive to cow's milk.
| Present symptoms during DBPCOFC to cow's milk | Percentage n=15 | 95%CI |
|---|---|---|
| Abdominal | 73.3% | 48.0–89.1% |
| Skin | 80.0% | 54.8–92.9% |
| Respiratory | 26.6% | 10.9–51.9% |
| Cardiovascular | 0% | NV |
| Anaphylaxis | 0% | NV |
DBPCFC were performed to 49 patients with suspected CMPA, the results presented here are those obtained in patients in whom the diagnosis was confirmed, for the remaining patients CMPA was discarded and they currently consume cow's milk protein without presenting any symptoms. NV: not valid.
The 15 patients with diagnosed CMPA were randomised to receive one of the two interventions (Fig. 1). All of the patients concluded the protocol. None of the 15 patients presented any symptoms from the amino-acid formula or from the camel's milk during the days the trial was carried out, hence they followed the free daily intake of 200ml of camel's milk over the following seven days.
None of the patients presented late adverse effects from ingesting camel's milk.
DiscussionThe prevalence of CMPA is a significant public health issue that has increased over the last decades,2 and whose treatment implies the total suspension of the intake of this protein. The clinical use of milk originating from other mammals has been proposed, including that of sheep, goats, donkeys and buffalo, however, their protein sequences have been found to be homologous to that of cow's milk, which is why their use is not recommended in CMPA patients.2 Camel's milk has been suggested as an alternative for this population due to its differences from cow's milk protein19 as well as its high nutritional content; camel's milk has been widely distributed and accepted in countries in East Africa for decades, and with the growing levels of urbanisation, consumption and commercialisation have increased in urban areas, improving quality standards, and managing sales in countries far from the Middle East.20–22
We did not find mediated or non-mediated IgE symptoms regarding the intake of camel's milk, proving a similar tolerability level between the amino-acid formula and camel's milk in CMPA patients, as found by El-Agamy et al.,16 who administered camel's milk to a group of 28 children with confirmed CMPA without any secondary clinical manifestations in its use; also Shabo et al.,17 who through a simple trial administered camel milk to a group of eight children with CMPA, proving adequate tolerance. Ehlayel et al. studied a group of 35 patients with symptoms related to CMPA with specific IgE and positive skin tests for cow's milk; all patients underwent skin tests for camel's milk and those with negative results (n=28) were administered camel's milk. None of those 28 patients presented any symptoms while undergoing the open trial.18
Throughout the ample literature review we carried out, we found only one reported case where a patient with CMPA suffered from anaphylaxis with camel's milk, published by Al-Hammadi23 in 2010, concerning an atopic six-year-old child with uncontrolled asthma, multiple food allergies and atopic dermatitis; we considered it unwise to carry out DBPCFCs with camel's milk on patients with uncontrolled allergic pathologies, especially with asthma, due to the high risk of severe side effects.
It is worth noting that as suggested in worldwide literature, we found a significant overdiagnosis of CMPA, which was only confirmed in 30.6% of patients who suspected suffering from CMPA.
Having an alternative to cow's milk for children with CMPA, which has an adequate taste and is safe to consume, is extremely important, as it could have a positive impact on the quality of life of patients and their families. Up until now, our study is the only one to have been carried out with DBPCFCs to value the tolerability of camel's milk in patients with CMPA, significantly reducing methodological bias and adding value to the presented results.
Although other authors have performed skin tests or measured camel's milk's specific IgE, we did not carry out those tests because we used DBPCFC, which is the gold standard of diagnosis and capable of diagnosing both IgE and non-IgE CMPA.
ConclusionOur study proves that camel's milk is safe and adequately tolerable for patients over the age of one with CMPA and could be considered as a good alternative due to its better flavour and lower cost than other formulas.
FundingCamel's milk was obtained as a donation from the Emirate company “Camelicious” Emirates Industry for Camel Milk & Products.
Conflict of interestThe authors declare no conflict of interest.
The authors thank “Camelicious” Emirates Industry for Camel Milk & Products for supplying camel milk and to Muhammad Ashraf, Sales Manager, who was personally in charge of the process.