Patients with a history of beta-lactam antibiotic allergy are often admitted to the hospital with severe or life-threatening infections requiring beta-lactam antibiotics. Strict avoidance of beta lactams to such patients may prevent them from getting adequate coverage and can lead to an increase in the use of alternative antibiotics, which can predispose to antibiotic resistance. Past studies revealed a lower incidence of pen allergy then patients’ histories suggest. Fortunately today, there are three options for patients presenting with a history of beta-lactam allergy. Penicillin skin testing, beta-lactam challenge or beta-lactam desensitization.
Recently Pre Pen has been FDA re-approved and when combined with Pen G is a valid way to determine if patients are able to tolerate beta-lactam antibiotic. When these agents are not available one must decide about desensitization or challenge. When a patient has a positive penicillin skin test, desensitization or beta-lactam avoidance are the only options.
This paper reviews the safety of beta-lactam desensitization.
ObjectiveTo perform a chart review on patients desensitised with beta lactam to determine if desensitizations can be performed safely without minimal complications.
MethodsA retrospective chart review was performed on allergy and immunology inpatient consultations for beta-lactam desensitization between September 2003 and August 2006 at the Cedars-Sinai Medical Centre in Los Angeles. Patient data and outcomes of desensitization were analysed.
ResultsA total of 13 intravenous desensitizations were performed on 12 patients. The patients consisted of eight females and four males with an average age of 65 years. Age range was 36–92 years old. All 13 intravenous desensitizations were completed without complications. No patient required a slower rate of desensitization or discontinuance of the desensitization. Patients were able to tolerate the initial therapeutic dose of their beta-lactam antibiotic and were then able to complete full therapeutic courses of their antibiotic.
ConclusionBeta-lactam antibiotic sensitivity continues to present a challenging problem for physicians. Patients with drug resistant infections who are unable to obtain skin testing or who test positive to skin tests may need either a challenge or desensitization. Desensitization, saved for those with a convincing beta-lactam hypersensitivity history is often the choice of last resort given the associated cost and risk of anaphylaxis. However, once desensitization is complete, patients are usually able to tolerate full doses of antibiotics for full treatment length with minimal side effects.
Beta-lactam antibiotics include the penicillin (PCN), cephalosporin, carbapenem and monobactam categories. When patients require these antibiotics for infection, avoidance of this large group of antibiotics is a challenge. In addition, many patients have drug resistant infections and do not have many antibiotic choices. For example, extended spectrum beta lactamase (ESBL) infections have only carbapenems as their treatment option. Currently, many physicians would avoid all beta-lactams including carbapenems in PCN allergic patients. This would leave patients with ESBL infections without a treatment option.
Patients with a clinical history of penicillin allergy upon hospitalization are much more likely to receive alternative antibiotics such as vancomycin and flouroquinolones.
This may attribute to an increase in vancomycin-resistant enterococci and fluoroquinolone-resistant pseudomonas.1
Excluding the monobactam category, all of the beta-lactam antibiotics share a common bicyclic core structure, the beta-lactam ring that is thought to be the basis for the cross-reactivity. The risk of cross reaction of these groups of antibiotics has a varied rate in the literature. According to studies, PCNs and cephalosporins have a cross-reactivity rate between 1 and 8%2 with first and second generations of cephalosporins having a higher rate.3 PCN and the monobactam aztreonam have no significant cross reactivity likely due to the fact that aztreonam has a monocyclic core structure.4 Aztreonam may have some cross reactivity to ceftazidine since they share a similar side chain.4 Data have shown some cross reaction among penicillin and amoxicillin and certain cephalosporins with similar side chains.5 Cross reactions among cephalosporins may also be due to these side chains.5 Finally, studies show conflicting data in the cross reactivity of PCNs and the carbapenems. Early studies have reported a higher rate of cross reaction up to 47%,6 but further studies have shown a much lower rate of 5–9%7 or an even lower rate that is described as minimal.8 The exact rate of cross reactivity between PCN and carbapenems is unknown.
Many patients have a history of penicillin allergy. Up to 10% of hospitalized patients report an allergy to PCN.9 However, many patients have only a vague history of PCN allergy or they report adverse effects mistakenly as an allergy.
Retrospective and prospective studies have shown a significant false-positive rate for self-reported penicillin allergy.10–12 Studies also show many patients lose their PCN allergy with only 30% maintaining their positive skin test at 10 years.9 Therefore it can be difficult to determine whether a patient is truly PCN allergic or not based solely on history.
Typical symptoms of PCN IgE-mediated allergic reaction include skin reactions, urticaria, angioedema, laryngeal edema, bronchospasm, and hypotension/shock. A thorough history can help differentiate adverse effects versus an IgE-mediated reaction.
It is also important to determine if a patient suffered from a hypersensitivity (IgE) reaction vs. another immunologic cellular reaction such as SJS/TENS. Patients presenting with a history of beta-lactam antibiotics causing blisters and burns may have experienced SJS or TENS life-threatening conditions where cell death leads to the separation of epidermis from the dermous. Beta-lactam skin testing or desensitization is contraindicated in these patients.
MethodsInpatient allergy and immunology consultations for beta-lactam desensitization between September 2003 and August 2006 at Cedars-Sinai Medical Centre in Los Angeles. Institutional Review Board approval was obtained. The allergy clinic received a total of 13 consultations for possible antibiotic desensitization of this period of time. All patients had a history consistent with beta-lactam allergy and had a serious infection requiring a beta-lactam antibiotic as their only treatment option. No PCN skin testing was performed, due to unavailability of the reagents at the time of admission. All patients were evaluated and referred by an infectious disease physician to a specific allergist/immunologist. All patients were evaluated and treated by the same allergist. No patients had a history of Steven-Johnson syndrome, toxic epidermal necrolysis, or penicillin-induced exfoliative dermatitis. Data collected included age, gender, admitting diagnosis, beta-lactam allergy history, desensitization complications and outcome.
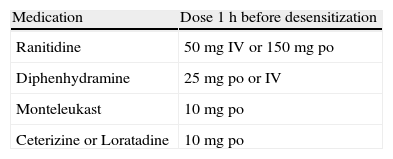
Patients were transferred to the intensive care unit for increased observation and for immediate access to emergency treatment if necessary. Patients were premedicated 1h before starting desensitization with the medications listed in Table 1. Beta-blocker medication was discontinued for at least 24h. All patients were informed of the risks/benefits of desensitization and consent forms were signed. Documentation was made in the patient's chart regarding the need for a beta-lactam antibiotic by the patient's primary care/critical care and infectious disease physicians.
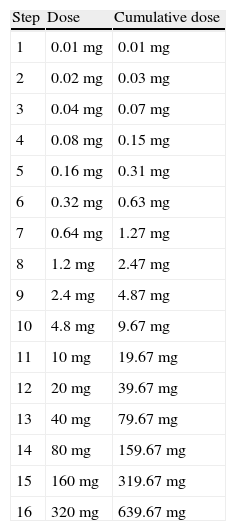
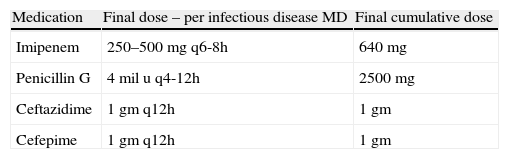
Desensitization occurred at 15min time intervals. Doses started at 0.1mg and were doubled at each interval until therapeutic doses were achieved (Table 2). Doses were infused intravenously in 50cc of normal saline over 30min. Medication was dispensed and mixed by the hospital pharmacy and the medication was administered by critical care nurses. The final antibiotic therapeutic dose and frequency was determined by the patient's infectious disease physician (Table 3). A physician was on premises at all times, but not at the patient's bedside. The patient's vital signs were monitored continuously including monitoring for anaphylaxis such as hypotension, tachycardia, and dyspnoea. Also every 15min the patient was observed for signs of allergic reaction including pruritus, rash or flushing, urticaria, angioedema, and wheezing. If flushing, a rash, or urticaria developed, a slower protocol of desensitization was to be used, and desensitization was to be stopped for angioedema, changes in respiratory status, or hypotension. Treatment for potential anaphylaxis was immediately available at bedside (Table 4).
Desensitization protocol performed in reviewed patients.
| Step | Dose | Cumulative dose |
| 1 | 0.01mg | 0.01mg |
| 2 | 0.02mg | 0.03mg |
| 3 | 0.04mg | 0.07mg |
| 4 | 0.08mg | 0.15mg |
| 5 | 0.16mg | 0.31mg |
| 6 | 0.32mg | 0.63mg |
| 7 | 0.64mg | 1.27mg |
| 8 | 1.2mg | 2.47mg |
| 9 | 2.4mg | 4.87mg |
| 10 | 4.8mg | 9.67mg |
| 11 | 10mg | 19.67mg |
| 12 | 20mg | 39.67mg |
| 13 | 40mg | 79.67mg |
| 14 | 80mg | 159.67mg |
| 15 | 160mg | 319.67mg |
| 16 | 320mg | 639.67mg |
This table was published in “Allergy Principles and Practice” 4th edition under the title “Protocol for parenteral desensitization of B-lactam antibiotic allergic patients” page 1738, copyright Elsevier 1993.
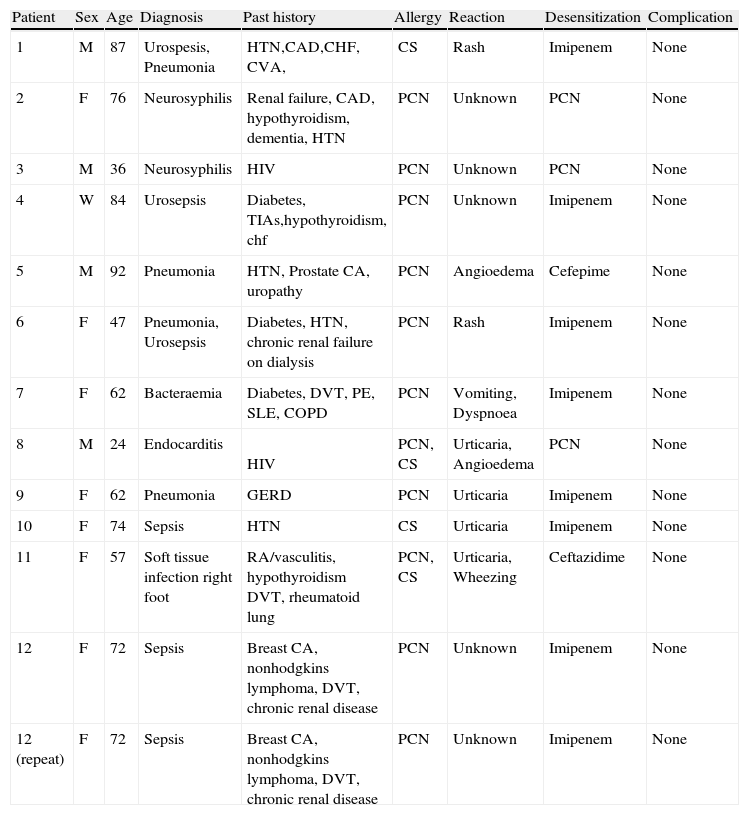
Desensitization patients co-morbidities.
| Patient | Sex | Age | Diagnosis | Past history | Allergy | Reaction | Desensitization | Complication |
| 1 | M | 87 | Urospesis, Pneumonia | HTN,CAD,CHF, CVA, | CS | Rash | Imipenem | None |
| 2 | F | 76 | Neurosyphilis | Renal failure, CAD, hypothyroidism, dementia, HTN | PCN | Unknown | PCN | None |
| 3 | M | 36 | Neurosyphilis | HIV | PCN | Unknown | PCN | None |
| 4 | W | 84 | Urosepsis | Diabetes, TIAs,hypothyroidism, chf | PCN | Unknown | Imipenem | None |
| 5 | M | 92 | Pneumonia | HTN, Prostate CA, uropathy | PCN | Angioedema | Cefepime | None |
| 6 | F | 47 | Pneumonia, Urosepsis | Diabetes, HTN, chronic renal failure on dialysis | PCN | Rash | Imipenem | None |
| 7 | F | 62 | Bacteraemia | Diabetes, DVT, PE, SLE, COPD | PCN | Vomiting, Dyspnoea | Imipenem | None |
| 8 | M | 24 | Endocarditis | HIV | PCN, CS | Urticaria, Angioedema | PCN | None |
| 9 | F | 62 | Pneumonia | GERD | PCN | Urticaria | Imipenem | None |
| 10 | F | 74 | Sepsis | HTN | CS | Urticaria | Imipenem | None |
| 11 | F | 57 | Soft tissue infection right foot | RA/vasculitis, hypothyroidism DVT, rheumatoid lung | PCN, CS | Urticaria, Wheezing | Ceftazidime | None |
| 12 | F | 72 | Sepsis | Breast CA, nonhodgkins lymphoma, DVT, chronic renal disease | PCN | Unknown | Imipenem | None |
| 12 (repeat) | F | 72 | Sepsis | Breast CA, nonhodgkins lymphoma, DVT, chronic renal disease | PCN | Unknown | Imipenem | None |
PCN, penicillin; CS, cephalosporin; F, female; M, male.
Thirteen intravenous desensitizations were completed on 12 patients; one patient was desensitised twice during different hospitalizations. Eight imipenem desensitizations, three penicillin desensitizations, and two cephalosporin desensitizations were performed. The patients consisted of eight females and four males with an average age of 65 years. Age range was 36–92 years old. No patients had a history of allergic rhinitis, asthma, or other atopic disease. Seven patients reported multiple drug allergies in addition to their beta-lactam allergy including sulfonamides, quinolones, tetracyclines, macrolides, telithromycin, vancomycin, terbinafine, cortisone, and iron.
All 13 intravenous desensitizations were completed without complications. No patients required a slower desensitization protocol or discontinuance of their desensitization. Patients were able to tolerate the therapeutic dose of their beta-lactam antibiotic and were able to complete full therapeutic courses of their antibiotic. No patient had a lapse in their treatment. Two patients after the desensitization, died later during their hospital course at a sufficient time after the desensitization. To conclude, their deaths were due to their underlying infection and were unrelated to the desensitization.
DiscussionIn beta-lactam allergic patients, desensitization is a procedure that can lead to decreased antibiotic restrictions by allowing the use of beta-lactam antibiotics. The mechanism of desensitization is unclear but is thought to be due to antigen-specific mast cell desensitization. Desensitization is only used in type-1 hypersensitivity reactions, those that are immediate and IgE-mediated. Desensitization is generally successful, in a retrospective review of 57 cases, 75% had successful desensitizations.13 Of the 11 cases in this review where desensitization failed due to allergic reactions, seven of the allergic reactions were not solely IgE-mediated. In another study, up to 30% of 26 patients had adverse effects such as pruritus and urticaria, but all patients were still able to tolerate complete desensitization.14 Our experience with 13 inpatient beta-lactam desensitizations had a decreased rate of adverse effects, possibly due to the smaller number of patients.
Our success in desensitization and lack of adverse effects raises the question that perhaps our patients were not beta-lactam allergic, had lost their beta-lactam allergy over time, or did not have an allergic cross-reactivity to their desensitised antibiotic.
Due to the lack of penicillin skin testing at the time of these patient admissions and due to the risk of possible anaphylaxis and/or death, desensitising these patients with a life-threatening infection with a beta-lactam allergy history was deemed necessary.
Skin testing with antibiotics other than PCN is not standardised, the negative predictive value of these skin tests is unknown, and the positive predictive value is unknown. Also, many of the beta-lactam antibiotics have metabolites that are major causes of allergic reactions. For example, in carbapenems the metabolised component of the drug is unavailable for skin testing and is a main trigger in immediate hypersensitivity reactions. Skin testing without the metabolised component could cause a false negative skin test.
In vitro tests for beta-lactam allergy are commercially available for PCN G, PCN V, ampicillin, amoxicillin and the second generation cephalosporin Cefaclor. These allergen specific IgE assays include the radioallergosorbent test (RAST) and Immunocap assay. Currently, using penicillin specific IgE assays to rule out a penicillin allergy is not recommended. However, a positive RAST or ImmunoCap test could be indicative of the presence of IgE antibodies and the patient would require desensitization of the needed antibiotic. RAST/Immmunocap testing has been shown to have a high specificity but unfortunately a low sensitivity compared to skin testing.15,16 Also, RAST testing has been shown in one study to only test for the major determinant.15 A more recent study failed to show the IgE antipenicillin fluorometric enzyme immunoassay as being useful in patients with remote histories of penicillin allergy.17 Thus, in vitro testing has significant limitations in determining true allergy.
The current commercial anti-penicillin IgE FEIAs are not useful in diagnosing penicillin allergy in patients with remote histories of penicillin allergy. Penicillin skin testing and, if the results are negative, an oral challenge remain the criterion standard tests to determine therapeutic penicillin tolerance.
Skin testing is clearly the preferred test for PCN allergy and has a negative predictive value of approximately 99% and a positive predictive value of 40–60%.18–21 In the early 1970s the major determinant to penicillin, termed pre pen was marketed. In the mid 2000s, pre pen was not available although recently Pre Pen has been re-approved and is currently available. Penicillin when metabolised consists of major determinants, which are usually responsible for less severe hypersensitivity reactions and minor determinants which are responsible for 20% of the more severe reactions.22 It is best to use both a minor and a major determinant for skin testing. Pen G has been found to contain these minor determinants.23
An important point to consider in desensitization is cost. Desensitization increases patient care costs by requiring ICU space as well as increased monitoring and nursing, physician, and pharmacist time.
This is why it is important to have skin testing with Pre Pen and Pen G available at facilities and to use desensitization when penicillin skin testing is positive. The above study along with others does show that desensitization is tolerated in the majority of patients. It would be worth determining the safety of pen desensitization in only pen positive patients now that skin testing is available.
Overall, Beta-lactam antibiotic allergies present a challenging problem for physicians. Patients with drug resistant infections whose antibiotic choice is limited may need desensitization. Desensitization is a choice of last resort given its associated cost and risk of anaphylaxis. Desensitization should be performed in the ICU setting for increased monitoring and should be performed by an experienced clinician. Once desensitization has been performed, patients may be able to tolerate full doses of antibiotics for the full length of treatment for their infection with minimal side effects.
Now that pen skin testing available, desensitization should be only required for patients who have positive penicillin skin tests. Future studies will be able to determine the safety/success in desensitization with skin test positive patients.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Conflicts of interestThe contents do not represent the views of the Department of Veterans Affairs or the United States Government.
This material is based upon work supported by the Department of Veterans Affairs.