Control cannot be achieved in some asthmatics although optimal monitoring and treatment is administered. Glucocorticoid (GC) resistance is one of the reasons of poor asthma control. We aimed to investigate GC resistance by lymphocyte proliferation suppression test (LPST) in uncontrolled asthmatics.
MethodsAfter assessing asthma control level of 77 asthmatics their treatment was adjusted upon GINA guidelines. They were followed-up for three to six months and the patients who remained uncontrolled were accepted as uncontrolled patients. Steroid resistance test (SRT) was applied to them (7–14 days oral prednisolone) and the patients who were still uncontrolled and/or had a FEV1 increase <15% after SRT were assessed as the “case group” while the remaining composed the “control group”. Optimal treatment was adjusted and at the end of a follow-up period LPST was performed to both groups.
ResultsFourteen of the case (n=22) and four (n=8) of the control groups could be evaluated by LPST. Proliferated lymphocytes were observed to be significantly suppressed in all dexamethasone concentrations in the control group (p=0.001). However, in the case group LPST was positive only at 10−6 and 10−4 concentrations although statistically not significant (p=0.147). There was no significant relationship between clinically GC resistance and LPST positivity (p=0.405).
ConclusionWe determined that in vitro responses to the GCs were significantly declined in the uncontrolled asthma cases. An SRT alone does not seem to be very sensitive for evaluating GC sensitivity, LPST may be performed for demonstrating GC responsiveness in asthmatic patients in addition to SRT.
Asthma prevalence has been reported between 1 and 18% across the world. Chronic inflammation and bronchial hyperreactivity are two major components of asthma which contribute to the development of the clinical aspects of the disease. Uncontrolled asthma is associated with significant disability and mortality in patients suffering from the disease. Therefore the goal in asthma therapy has been defined as achieving disease control and patients are classified into three groups as “controlled”, “partly controlled” and “uncontrolled” according to their control status.1 Several factors including poor adherence to drug therapy, inadequate treatment and associated comorbidities may have an influence on asthma control. A small number of asthmatic patients could not be controlled even though optimal monitoring and treatment was administered. Approximately 30% of all asthmatic patients are uncontrolled despite high dose steroid therapy and 10% of the asthmatics are classified as severe asthmatics.2,3
It has been reported that problems in achieving asthma control might be related with drug bioavailability as well as underlying clinical challenges. Glucocorticoid (GC) resistance might be one of the underlying aetiologies of poor asthma control.4 GCs are strong anti-inflammatory drugs which activate and suppress many pro and anti inflammatory genes as well as having post-transcriptional effects. They are used both in asthma attacks and in the maintenance treatment.5 GC resistant (GC-R) cases do not respond to GCs for some duration or dose; they may need high doses of GCs and they are characterised with severe asthma phenotype.5,6 Two subtypes of GC resistance have been defined as type I and type II. In type I, the resistance is acquired from allergens, infections or chronic high dose beta agonist and/or GC use and the rest of the organism other than immune cells are sensitive to GCs. Type II GC resistance which is observed in 5% of GC-R cases is also known as familial cortisol resistance and affects all cell types in the organism. A genetic mutation has been suggested as being responsible for this irreversible GC resistance.5
Different studies have reported that T lymphocyte activation is correlated with the disease severity and GC therapy reduces peripheral blood T lymphocyte activation compatible with the clinical improvement.7,8 It has been demonstrated that in vitro responses to GC are decreased in circulating T-lymphocytes of GC-R asthmatic patients.9,10 In GC sensitive (GC-S) asthmatics, phytohaemagglutinin stimulated peripheral blood mononuclear cell proliferation has been found to be suppressed by GCs. However, this GC response could not be achieved in GC-R asthmatics, which supported the hypothesis of GC resistance in T lymphocytes and monocytes. It has been shown that secretion of interleukin (IL)-2 and interferon-γ from phytohaemagglutinin stimulated peripheral blood T lymphocytes are not inhibited in GC-R asthmatics.11 On the other hand, the degree of inhibition of lymphocyte proliferation has been found to be associated with the binding affinity of dexamethasone to the GC receptors. This finding supports the role of GC receptor activity in GC responses to phytohaemagglutinin stimulated lymphocyte proliferation.12
In our country, there is not enough data about the frequency of GC resistance in asthmatic patients who are not controlled even with the optimal asthma treatment. Therefore, we aimed to investigate the GC resistance by lymphocyte proliferation suppression test (LPST) in those asthmatic patients who were not controlled or clinically GC resistant.
Materials and methodSeventy-seven asthmatic patients admitted to our outpatient clinic between January 2008 and 2010 were screened for the study. All the patients with an asthma diagnosis for more than one year were included consecutively regardless of their asthma control level. Having major co morbid diseases, other overlapping pulmonary diseases (bronchiectasis, cord vocal dysfunction, chronic obstructive pulmonary disease), uncontrolled psychiatric disorders, ongoing occupational exposure and active smoking were the exclusion criteria. Local ethics committee approval was obtained and all patients signed informed consent.
All the enrolled patients were evaluated with a standard questionnaire for demonstrating demographical and historical data. Asthma control level was assessed according to GINA 2006 and treatment was adjusted upon the same guidelines for each control level.1 Comorbidities like allergic rhinitis and gastrooesophageal reflux were treated as needed. They were followed for minimum three, maximum six months. The patients who remained uncontrolled at the end of the treatment period were accepted as uncontrolled patients (n=22). They were receiving inhaled corticosteroids and long acting beta agonists and/or leukotriene receptor antagonists and/or theophylline in general. Steroid resistance test was applied to them (30–40mg/day per oral prednisolone for 7–14 days).13 These uncontrolled cases with or without a FEV1 increase <15% after steroid resistance test were assessed as the “case group” (n=22). The “control group” was composed from randomly selected controlled patients (n=50) and they were adjusted for optimal treatment and followed for three to six months. At the end of the follow up period LPST was performed to the enrolled case (n=22) and control (n=8) groups who approved to participate in the study.
Lymphocyte proliferation suppression testIsolation of peripheral blood lymphocytes and measurement of cell proliferationBlood samples were collected between 09.00 and 10.00a.m. in a heparinised Cell Preparation Tube (BD Vacutainer, TM) and centrifuged at 1800 RCF at room temperature for 15min in 2h. Approximately 2ml of the cloudy part was taken and centrifuged with plasma. Cell pellets were collected with a Pasteur pipette and transferred to 15ml falcon tubes. After washing procedures with PBS peripheral blood mononuclear cells were separated.
Cell viability was determined by Trypan blue staining (Gibco) and cells were counted on haemocytometer. Isolated lymphocytes were seeded in a 96-well flat bottom (dark) tissue culture plate at a concentration 1×105cell/well containing 200ml of RPMI-1640 (supplemented with 10%FCS and %1 Pen/Strep antibiotics). Cells were cultured in the presence of dexamethasone with or without phytohaemagglutinin (PHA, 0.006μg/μl). Optimal PHA concentration was chosen by titration studies. Appropriate stock solutions of dexamethasone were prepared and added to every well as to 10−4, 10−6, 10−8, 10−10M. Quantitative measurements of cell proliferation were assayed by BrdU ELISA assay (Roche). BrdU is an analogue of the DNA precursor thymidine. In proliferating cells, the DNA has to be replicated before the division takes place. The amount of BrdU in the DNA of cells can be detected with specific ELISA monoclonal antibodies against BrdU. According to the manufacturer's procedures, 10μl of BrdU solution was added to all the wells and incubated at 37°C for 24h for incorporation of BrdU to the newly synthesised DNA. After the incubation period culture medium was removed and all the wells were fixed and DNA was denatured with FixDenat solution of the kit. Anti-BrdU- POD antibody was added to the wells to bind denatured and newly synthesised cellular DNA. Addition of the substrate formed immune complex to quantify of DNA as light units/s (rlu/s) by luminometer (BioTek Instruments). The relative light units/second directly correlates to the amount of DNA synthesis and to the number of proliferating cells in the respective micro cultures.
Statistical analysisThe statistical analyses were done with SPSS 11.0 package. Two groups were compared by Mann–Whitney U test. Fisher's exact test was used for categorical analysis. For repeated measures Benferonni adjusted Friedman tests were used. p<0.05 was considered to be significant.
ResultsThere were 22 patients in the case group and 50 patients in the control group. All the patients in the case (n=22/22) and eight patients in the control group (n=8/50) approved to be involved in the study. Nine patients in the case group were found negative for steroid resistance test. Fourteen patients in the case group (mean age 53.5±12.7) and four patients in the control group (mean age 42.0±14.5) had specimens with lymphocytes having 95% viability and LPST was performed to them. The demographic and functional characteristics of these patients are shown in Table 1. The age, sex, smoking status, atopy, rhinitis and eosinophilia and comorbidity presence were similar in both groups (p=0.524, p=0.079, p=1.000, p=0.569, p=0.092, p=1.000, p=1.000, respectively). Daily short acting beta agonist (SABA) and inhaled corticosteroid (ICS) use and acute exacerbations which needed ICS therapy were observed to be more frequent in the control group (Table 1).
Demographic and functional characteristics of the study groups.
| CASE (n=14) | Control (n=4) | |
| Age (year) (mean±SD) | 53.5±12.7 | 42.0±14.5 |
| aGender (female/male) | 10/4 | 4/0 |
| aSmoking status (smoker/ex-smoker/non-smoker) | 0/5/9 | 0/1/3 |
| aAtopy history | 10 | 2 |
| aRhinitis presence | 8 | – |
| aPeripheral eosinophilia | 8 | 2 |
| aComorbidity | 4 | 1 |
| aFEV1/FVC (≤70%) | 7 | 0 |
| aFEV1% | ||
| 30–60% | 5 | 0 |
| 60–80% | 0 | 0 |
| >80% | 9 | 4 |
| Asthma diagnosis age (mean±SD) | 37.3±13.6 | 34.0±12.6 |
| Mean duration after asthma diagnosis (mean±SD) | 16.2±10.9 | 8±2.4 |
| Asthma control test score (mean±SD) | 12.2±4.9 | 20.2±3.1 |
| Number of exacerbations/year | 2.0 | 0.0 |
| aCombined LABA+ICS therapy | 14 | 4 |
| aLTA therapy | 13 | 2 |
| aTheophylline therapy | 9 | – |
| Daily ICS dose (μg/day BUD or equivalent) (mean±SD) | 823.57 | 312.5 |
| aRegular parenteral CS therapy | 13 | – |
| Daily SABA need (puff/day) (mean±SD) | 3.0 | 0.0 |
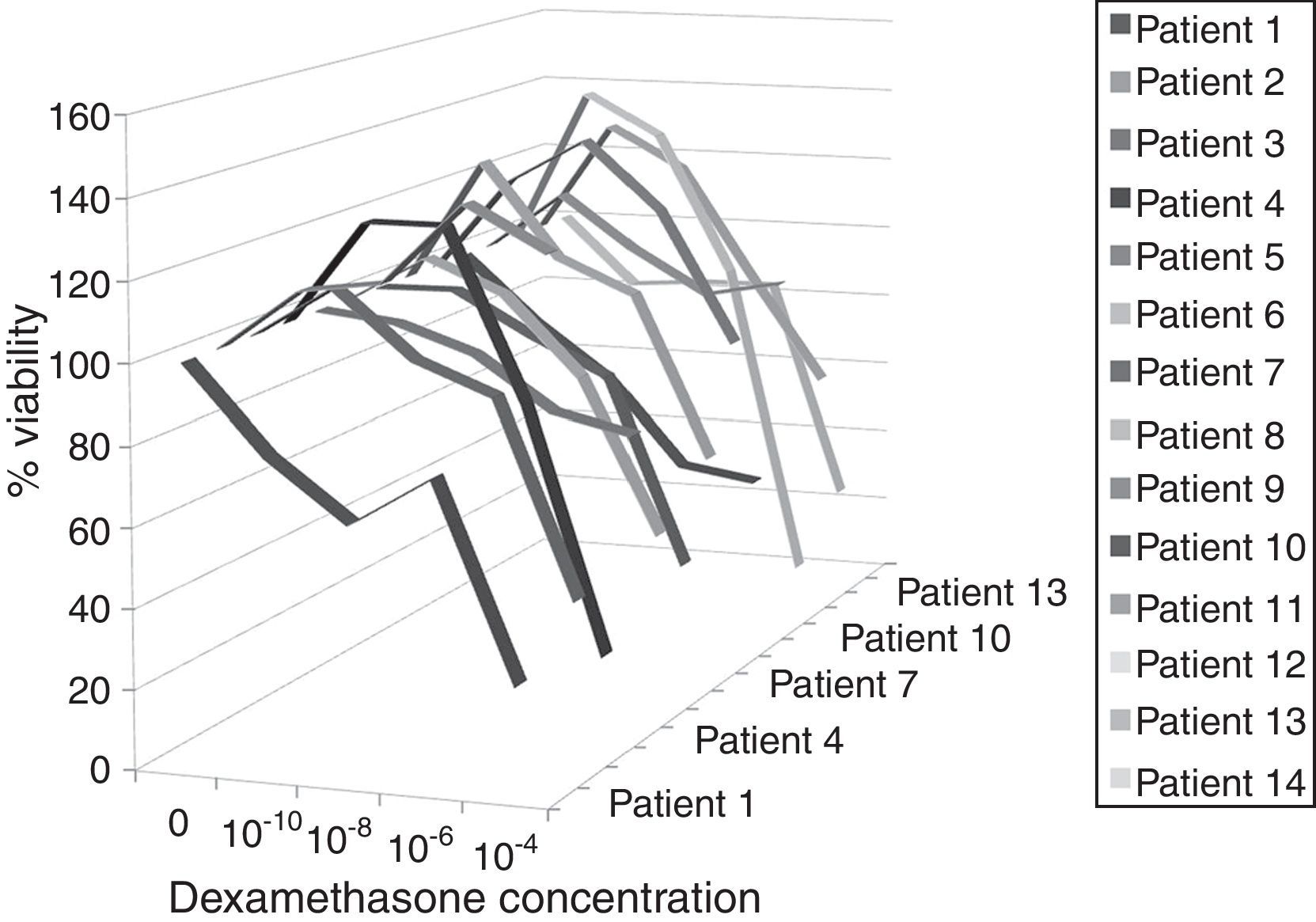
In the control group, different suppression levels were observed in proliferated lymphocytes at 10−10, 10−8, 10−6, 10−4mol concentrations of dexamethasone and suppression levels were statistically significant when repeated measurements were performed (p=0.001) (Fig. 1). The results of the analyses showed that dexamethasone in 10−4mol concentration suppressed lymphocytes more than other concentrations, while 10−6mol concentration made suppression bigger than 10−10, both statistically significant (p=0.04) (Table 2).
Lymphocyte viability for different dexamethasone concentrations in case and control groups.
| Dexamethasone concentration | CASE (n=14) | Control (n=4) | p |
| 0mol | 100.0±0.0 | 100.0±0.0 | 1.000 |
| 10−10mol | 110.6±20.2 | 29.9±22.8 | 0.003* |
| 10−8mol | 102.2±22.4 | 31.8±28.3 | 0.008* |
| 10−6mol | 88.5±21.2 | 15.1±19.8 | 0.008* |
| 10−4mol | 51.9±32.8 | 9.2±9.5 | 0.008* |
Values are shown as mean±standard deviation.
In the case group proliferated lymphocytes were observed to be suppressed by 10−6 and 10−4 dexamethasone concentrations, however suppression level was not statistically significant for these concentrations with respect to other concentrations (p=0.147) (Fig. 2). Furthermore, dexamethasone suppression was significantly lower in the case group for all concentrations with respect to the control group (p=0.003 for 10−10, p=0.008 for other concentrations (Table 2).
It was possible to perform LPST to nine patients among the 13 steroid resistance test positive patients in the case group. In five patients out of these nine; who were positive for steroid resistance test; lymphocyte vitality rate was more than 50% in 10−4mol concentration. The remaining nine patients were negative for steroid resistance test and five underwent LPST. However, among these five steroid resistance test negative patients four had lymphocyte vitality rates lower than 50% in 10−4mol concentration. There was no significant relationship between GC resistance and LPS presence (p=0.405).
DiscussionUncontrolled asthma is associated with significant disability and mortality in patients suffering from the disease. Therefore, we aimed to investigate the GC resistance by steroid resistance and lymphocyte proliferation suppression tests in the asthmatic patients who were classified as severe asthmatics or uncontrolled cases. Although we were able to include a small number of patients, we found that steroid resistance test alone was not very sensitive for evaluating GC response and that lymphocyte proliferation suppression test might have a value to demonstrate GC resistance in severe asthmatic patients.
The descriptive features of severe asthmatics include female gender, being over 40 years old and atopy. Occupational exposure, chronic rhinosinusitis, nasal polyposis and persistent eosinophilia despite high dose steroids are the other phenotypic characteristics of these patients.14–17 They usually present with frequent exacerbations and uncontrolled disease despite high dose ICS use and multiple drug therapies. Irreversible obstruction has been reported to be between 35 and 50% of the adult asthmatics and persistent eosinophilia under treatment might contribute to this procedure.17,18 In our study, the patients in the case group also had airflow obstruction. They were using high dose ICSs and needed more reliever drugs although their maintenance treatment included multiple drugs. Frequent exacerbations and systemic GC use were also observed in these cases. Therefore, the phenotypic features of our case group were compatible with severe asthmatics. However, as we could not determine GC resistance by steroid resistance test in five patients in the case group, we considered the participants in the case group a heterogeneous combination of different phenotypes. This finding supports the prior hypothesis suggesting that GC insensitive asthma should have not been limited with severe asthma.19 Even milder asthmatics might have GC resistance.11
It has been reported that T lymphocytes of clinically GC-R asthmatic patients also showed a relative unresponsiveness to the inhibitory effects of GCs.9,20,21 Haczku et al. found that dexamethasone addition to phytohaemagglutinin stimulated T lymphocytes in a range to 10−10 to 10−6 resulted in dose dependent suppression in both clinically GC-R and GC-S patients. They showed that T lymphocytes of GC-S patients were significantly suppressed in 10−6, 10−7 and 10−8mol concentrations with respect to GC-R patients.22 In another study, dose dependent suppression was reported in both GC-R and GC-S patients, albeit GC-R requiring more amount of GCs.12 In our study, lymphocyte proliferation was suppressed in every dexamethasone concentration we used in the control group. However, a decreased dexamethasone suppression response was observed for only 10−6 and 10−4 dexamethasone concentrations in the case group which composed from uncontrolled asthmatics. The differences in suppression degrees between the case and control groups were statistically significant. This finding suggests that responsiveness to GCs has been markedly impaired in uncontrolled asthmatics. Despite the fact that we did not start with an intention to demonstrate the possible mechanism of GC insensitivity, we determined that in nearly half of the clinically GC-R patients lymphocyte proliferation suppression was not efficient even with high doses of dexamethasone. We also determined marked lymphocyte proliferation suppression with dexamethasone in patients without GC resistance. Therefore we thought that there was type II GC resistance in some of the GC-R patients in our study group, while the other clinically GC-R patients possibly had type I resistance.
In conclusion, our results showed that in vitro responses to the GCs were significantly declined in the uncontrolled asthma cases. Steroid resistance test alone was not very sensitive for evaluating in vitro GC responses, as there were patients whose steroid resistance test was negative but peripheral blood lymphocyte proliferation was markedly suppressed. Therefore, in some clinically GC-R patients there could be type II GC resistance and LPST might be performed for demonstrating GC responsiveness in asthmatic patients in addition to steroid resistance test.
Ethical disclosuresPatients’ data protectionConfidentiality of Data. The authors declare that no patient data appears in this article.
Right to privacy and informed consentRight to privacy and informed consent. The authors declare that no patient data appears in this article.
Protection of human subjects and animals in researchProtection of human and animal subjects. The authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Financial supportDokuz Eylul University Scientific Research Fund, 200757.
Conflict of interestThe authors have no conflict of interest to declare.
The work was performed in Dokuz Eylul University Medical Faculty Pulmonary Diseases and Biochemistry Departments.