The accurate identification of sensitizing proteins in patients allergic to plant-derived foods is extremely important, allowing a correct dietary advice. We aimed to evaluate the diagnostic usefulness of skin prick tests (SPT) and specific IgE (sIgE) with single molecular allergen components in children with allergy to fruits and vegetables.
MethodsTwenty children underwent SPT with a palm profilin (Pho d 2, 50μg/mL); a Mal d 1-enriched apple extract (2μg/mL) (PR-10 allergen); and a peach Lipid Transfer Protein (LTP) (Pru p 3, 30μg/mL). Detection of sIgE to rBet v 1, rBet v 2, Phl p 12 and Pru p 3 was also measured.
ResultsAllergy to multiple fruits and vegetables was observed in 11 (55%) children. Sensitization by SPT to Pho d 2, Mal d 1, and Pru p 3 occurred in 5, 7, and 8 cases, respectively. LTP sensitization appeared to be associated with peach allergy but not with severe reactions, and profilins sensitization to melon and tomato allergy. Kiwi sensitization (12 cases), the plant-derived food that caused more allergic reactions, seemed mostly species-specific. The concordance of SPT extracts and sIgE to the corresponding pan-allergens was high for profilins (k=0.857) and LTP (k=0.706), while for PR-10 allergens it was absent (k=0.079).
ConclusionsPan-allergen sensitization in children with allergy to fruits and vegetables was common and often multiple. There was no association of severe reactions to LTP sensitization. The introduction of routine SPT to pan-allergens can be a simple and feasible way of improving diagnostic and therapeutic efficacy.
Food allergy is an important health problem affecting about 5–6% of children. Fruits and vegetables are one of the most commonly implicated foods in children over five years of age and adolescents.1
The diagnosis of allergy to fruits and vegetables is often complex since it involves various factors and co-factors that altogether strongly contribute to different clinical expressions of allergy. The stability of the allergens, which is dependent on their physical and chemical properties, is one of the most important aspects. Allergens that are stable to heat and digestion might induce potentially severe systemic symptoms, whereas heat and digestion-labile allergens are more likely to be tolerated or, instead, cause milder/local symptoms only, like the oral allergy syndrome (OAS).2,3
In patients with allergy to fruits and vegetables, three major groups of allergenic proteins from different species, but with high homology, have been identified. The most prominent are profilins, Pathogenesis-Related protein family 10 (PR-10) (homologous to Bet v 1), and the non-specific Lipid Transfer Proteins (LTP), which we call pan-allergens.4 In the Mediterranean area, the most frequent cross-reactivity syndromes are allergy to Rosaceae (mediated by LTP) without associated pollinosis, and the pollen-fruits syndrome mediated by profilins, with the absence of specific IgE (sIgE) to Bet v 1.5–7
The accurate identification of sensitizing proteins in patients allergic to plant-derived foods is therefore extremely important, since it will allow the recognition of cross-reactivity phenomena, the clinical relevance of allergens and the risk of severe reactions, thus enabling a correct dietary advice with a consequent improvement in patient's quality of life. The main shortcoming lies in the absence of simple and easy tools that can be used in daily clinical practice.
This diagnostic limitation has driven the development of molecular diagnostic tests, also referred to as component-resolved diagnostics (CRD).8 New extracts for skin prick tests (SPT) and new methods to measure sIgE have become commercially available during the past decade and have proven useful by overcoming the limitations of standard techniques.5,9,10 Therefore, the introduction of routine SPT to pan-allergens can be a simple and feasible way of improving diagnostic efficacy.
This study aimed to evaluate and compare the diagnostic usefulness of SPT and sIgE with single-molecular allergen components in children with allergy to fruits and vegetables.
Materials and methodsPatientsChildren seen at the Allergology Unit of the Centro Hospitalar do Porto, Portugal, with allergy to fruits and vegetables were consecutively selected. Food allergy was confirmed by a compatible clinical history, positive SPT with commercial food extracts and/or with fresh offending foods by prick–prick technique, sIgE when available, and open oral food challenges, when pertinent.
The inclusion criteria, which were considered compatible with food allergy, were: a history of OAS (defined as pruritus of the oral mucosa and lips with or without angio-oedema immediately after eating specific foods), urticaria with or without angio-oedema, respiratory symptoms, and/or of severe gastrointestinal disorders, following the ingestion of specific plant-derived foods. Children who received immunotherapy to pollens were excluded.
Latex allergy can be the cause of plant-derived food allergy, explained by cross-reactivity phenomena (latex-fruits syndrome). One child included in the present study had clinical manifestations compatible with latex allergy (apart from reactions with food), confirmed by positive SPT and sIgE by standard methods: commercial natural rubber latex extract (500μg protein/mL) and latex-sIgE by ImmunoCAP® (Phadia, Sweden). When two or more foods to which children were allergic belonged to the group of foods most frequently involved in the latex-fruit syndrome (banana, avocado, kiwi and tomato), latex allergy was investigated by SPT and sIgE by standard methods. Molecular assays were not conducted to latex.
All patients were located in the Mediterranean area, where seasonal allergy to grasses is predominant.
Patients’ baseline characteristics including demographic data and concomitant diseases (such as rhinitis, rhino-conjunctivitis, asthma and atopic dermatitis) were collected historically by means of a standardized interview and review of the relevant medical files.
Parents or legal guardians provided informed consent regarding each child's participation in the study.
Skin prick test extractsChildren underwent SPT with commercial extracts of mites, cat and dog dander, moulds and the most common pollens in the geographical area of the study, including grass, mugwort (Artemisia vulgaris), pellitory (Parietaria judaica), plantain (Plantago lanceolata), olive (Olea europaea), and birch (Betula alba) (Allergopharma, Germany). Furthermore, all patients underwent SPT with the three most relevant pan-allergen families in our geographic area (profilins, PR-10 allergens, and LTP).
A natural profilin (Pho d 2, 50μg/mL), was purified from a date palm extract.11
A PR-10 extract was obtained from a Mal d 1-enriched apple extract (2μg/mL).12 Mal d 1 exhibits a high homology with rBet v 1: 64.5% identity on the amino acid level and 55.6% identity on the nucleic acid level. Cross-reactivity between them was shown by inhibition assays.13
A peach LTP commercial extract, adjusted to 30mg/mL of Pru p 3 was used, and was shown to lack other relevant allergens (such as Pru p 1 and Pru p 4).14
The Pho d 2 and peach Pru p 3 extracts are commercially available, and the apple Mal d 1 extract has been used in the Europrevall study, and all were manufactured by ALK-Abelló, Spain.
Skin prick tests were performed by conventional procedures on the volar side of the forearm using disposable 1mm tip lancets (Stallergenes, France). Readings were taken after 15min. Reactions were expressed as the mean wheal diameter (adding the longest diameter to the orthogonal diameter and dividing by two). A mean wheal diameter of 3mm or more was considered positive. Skin prick tests with histamine 10mg/mL and saline solution were carried out as positive and negative control, respectively.15
Molecular allergens for specific immunoglobulin EDetection of sIgE to Betula verrucosa rBet v 1 and rBet v 2, Phleum pratense rPhl p 12, and Prunus persica rPru p 3 were measured in all patients by using the ImmunoCAP® system, according to the manufacturer's instructions. Levels greater than 0.35kU/L were considered positive.
In the light of previous studies showing that the sensitivity of rBet v 2 is not ideal in the detection of subjects hypersensitive to profilins,16 a sIgE to rPhl p 12 was additionally assayed.17 A positive sIgE to rBet v 1 was adopted as a marker of sensitization to PR-10 allergens.16 Specific IgE to Pru p 3 showed the best sensitivity (88.0%) and specificity (100.0%) out of four techniques for LTP sensitization,18 thus becoming our selection for this study.
Statistical methodsFor each variable, we used standard methods to calculate proportions, means and standard deviations (SD).
Odds ratio (OR) estimates were used to evaluate the strength of association between allergy to multiple fruits and vegetables and sensitization to tested pan-allergens by SPT.
The agreement between the two diagnostic techniques was analyzed using the κ statistic. Variables were expressed dichotomously as positive–negative in order to simplify the interpretation of the results.
Other qualitative and quantitative variables were compared using the Chi-square or Mann–Whitney–Wilcoxon non-parametric tests, when appropriate.
The results were considered statistically significant when p-values were <0.05. Data were analysed using the IBM SPSS® v.19.0 statistical package.
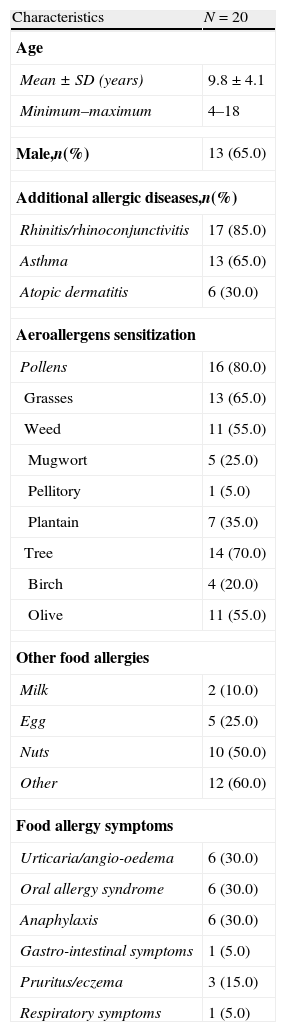
ResultsPatients’ baseline characteristicsTwenty children (65% male and mean age of 9.8±4.1 years, ranging from 4 and 18 years) were studied. Their grouped baseline characteristics are shown in Table 1, and the individual demographic, clinical, and allergenic characterisation in Table 2.
Characteristics of the study population.
| Characteristics | N=20 |
| Age | |
| Mean±SD (years) | 9.8±4.1 |
| Minimum–maximum | 4–18 |
| Male,n(%) | 13 (65.0) |
| Additional allergic diseases,n(%) | |
| Rhinitis/rhinoconjunctivitis | 17 (85.0) |
| Asthma | 13 (65.0) |
| Atopic dermatitis | 6 (30.0) |
| Aeroallergens sensitization | |
| Pollens | 16 (80.0) |
| Grasses | 13 (65.0) |
| Weed | 11 (55.0) |
| Mugwort | 5 (25.0) |
| Pellitory | 1 (5.0) |
| Plantain | 7 (35.0) |
| Tree | 14 (70.0) |
| Birch | 4 (20.0) |
| Olive | 11 (55.0) |
| Other food allergies | |
| Milk | 2 (10.0) |
| Egg | 5 (25.0) |
| Nuts | 10 (50.0) |
| Other | 12 (60.0) |
| Food allergy symptoms | |
| Urticaria/angio-oedema | 6 (30.0) |
| Oral allergy syndrome | 6 (30.0) |
| Anaphylaxis | 6 (30.0) |
| Gastro-intestinal symptoms | 1 (5.0) |
| Pruritus/eczema | 3 (15.0) |
| Respiratory symptoms | 1 (5.0) |
Data are presented as mean±standard deviation (SD) or N (%), unless otherwise indicated.
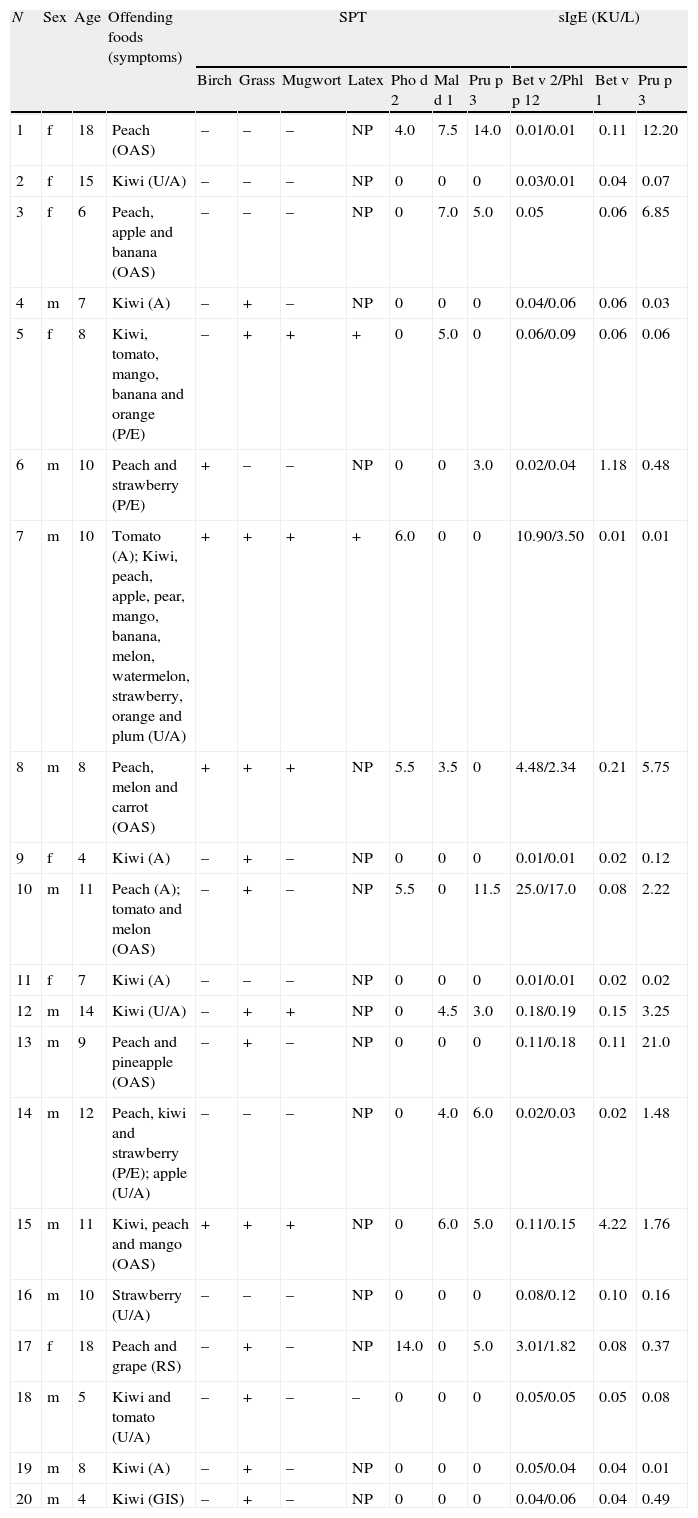
Demographic, clinical, and allergenic characterization of tested children.
| N | Sex | Age | Offending foods (symptoms) | SPT | sIgE (KU/L) | ||||||||
| Birch | Grass | Mugwort | Latex | Pho d 2 | Mal d 1 | Pru p 3 | Bet v 2/Phl p 12 | Bet v 1 | Pru p 3 | ||||
| 1 | f | 18 | Peach (OAS) | – | – | – | NP | 4.0 | 7.5 | 14.0 | 0.01/0.01 | 0.11 | 12.20 |
| 2 | f | 15 | Kiwi (U/A) | – | – | – | NP | 0 | 0 | 0 | 0.03/0.01 | 0.04 | 0.07 |
| 3 | f | 6 | Peach, apple and banana (OAS) | – | – | – | NP | 0 | 7.0 | 5.0 | 0.05 | 0.06 | 6.85 |
| 4 | m | 7 | Kiwi (A) | – | + | – | NP | 0 | 0 | 0 | 0.04/0.06 | 0.06 | 0.03 |
| 5 | f | 8 | Kiwi, tomato, mango, banana and orange (P/E) | – | + | + | + | 0 | 5.0 | 0 | 0.06/0.09 | 0.06 | 0.06 |
| 6 | m | 10 | Peach and strawberry (P/E) | + | – | – | NP | 0 | 0 | 3.0 | 0.02/0.04 | 1.18 | 0.48 |
| 7 | m | 10 | Tomato (A); Kiwi, peach, apple, pear, mango, banana, melon, watermelon, strawberry, orange and plum (U/A) | + | + | + | + | 6.0 | 0 | 0 | 10.90/3.50 | 0.01 | 0.01 |
| 8 | m | 8 | Peach, melon and carrot (OAS) | + | + | + | NP | 5.5 | 3.5 | 0 | 4.48/2.34 | 0.21 | 5.75 |
| 9 | f | 4 | Kiwi (A) | – | + | – | NP | 0 | 0 | 0 | 0.01/0.01 | 0.02 | 0.12 |
| 10 | m | 11 | Peach (A); tomato and melon (OAS) | – | + | – | NP | 5.5 | 0 | 11.5 | 25.0/17.0 | 0.08 | 2.22 |
| 11 | f | 7 | Kiwi (A) | – | – | – | NP | 0 | 0 | 0 | 0.01/0.01 | 0.02 | 0.02 |
| 12 | m | 14 | Kiwi (U/A) | – | + | + | NP | 0 | 4.5 | 3.0 | 0.18/0.19 | 0.15 | 3.25 |
| 13 | m | 9 | Peach and pineapple (OAS) | – | + | – | NP | 0 | 0 | 0 | 0.11/0.18 | 0.11 | 21.0 |
| 14 | m | 12 | Peach, kiwi and strawberry (P/E); apple (U/A) | – | – | – | NP | 0 | 4.0 | 6.0 | 0.02/0.03 | 0.02 | 1.48 |
| 15 | m | 11 | Kiwi, peach and mango (OAS) | + | + | + | NP | 0 | 6.0 | 5.0 | 0.11/0.15 | 4.22 | 1.76 |
| 16 | m | 10 | Strawberry (U/A) | – | – | – | NP | 0 | 0 | 0 | 0.08/0.12 | 0.10 | 0.16 |
| 17 | f | 18 | Peach and grape (RS) | – | + | – | NP | 14.0 | 0 | 5.0 | 3.01/1.82 | 0.08 | 0.37 |
| 18 | m | 5 | Kiwi and tomato (U/A) | – | + | – | – | 0 | 0 | 0 | 0.05/0.05 | 0.05 | 0.08 |
| 19 | m | 8 | Kiwi (A) | – | + | – | NP | 0 | 0 | 0 | 0.05/0.04 | 0.04 | 0.01 |
| 20 | m | 4 | Kiwi (GIS) | – | + | – | NP | 0 | 0 | 0 | 0.04/0.06 | 0.04 | 0.49 |
A – anaphylaxis; f – female; m – male; OAS – oral allergy syndrome; P/E – pruritus or eczema; sIgE – specific IgE; SPT – skin prick tests; U/A – urticaria and/or angio-oedema; GIS – isolated gastro-intestinal symptoms; NP – not performed; RS – isolated respiratory symptoms. SPT results for birch, grass, mugwort and latex are shown as positive (+) or negative (−), and for Pho d 2, Mal d 1 and Pru p 3 as mean wheal diameters (mm).
Although the patients were selected exclusively on the basis of food allergy, a significant number of children reported respiratory allergy: 17 (85%) had rhinitis with or without conjunctivitis, and 13 (65%) had asthma. Six (30%) children had atopic dermatitis.
Grass allergy was the most frequent seasonal sensitization, presenting in 13 (65%) children. Olive sensitization ranked second in prevalence with 11 patients (55%) sensitized, followed by plantain in seven (35%) cases.
Clinical presentation of food allergyThe offending foods reported by patients are shown in Tables 2 and 3.
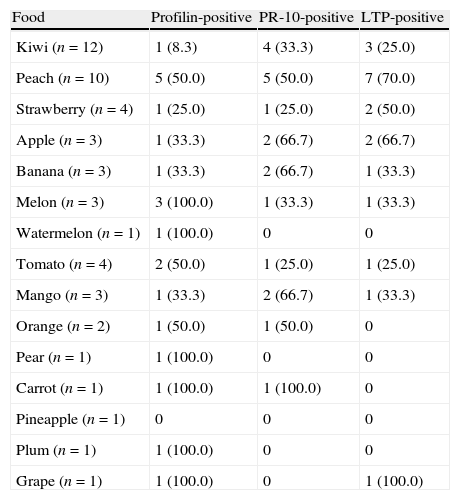
Offending foods and sensitization to the pan-allergens tested by SPT.
| Food | Profilin-positive | PR-10-positive | LTP-positive |
| Kiwi (n=12) | 1 (8.3) | 4 (33.3) | 3 (25.0) |
| Peach (n=10) | 5 (50.0) | 5 (50.0) | 7 (70.0) |
| Strawberry (n=4) | 1 (25.0) | 1 (25.0) | 2 (50.0) |
| Apple (n=3) | 1 (33.3) | 2 (66.7) | 2 (66.7) |
| Banana (n=3) | 1 (33.3) | 2 (66.7) | 1 (33.3) |
| Melon (n=3) | 3 (100.0) | 1 (33.3) | 1 (33.3) |
| Watermelon (n=1) | 1 (100.0) | 0 | 0 |
| Tomato (n=4) | 2 (50.0) | 1 (25.0) | 1 (25.0) |
| Mango (n=3) | 1 (33.3) | 2 (66.7) | 1 (33.3) |
| Orange (n=2) | 1 (50.0) | 1 (50.0) | 0 |
| Pear (n=1) | 1 (100.0) | 0 | 0 |
| Carrot (n=1) | 1 (100.0) | 1 (100.0) | 0 |
| Pineapple (n=1) | 0 | 0 | 0 |
| Plum (n=1) | 1 (100.0) | 0 | 0 |
| Grape (n=1) | 1 (100.0) | 0 | 1 (100.0) |
Data are presented as N (%). LTP – Lipid Transfer Proteins; PR-10 – Pathogenesis-Related protein family 10.
Kiwi was the plant-derived fresh food which most frequently elicited allergic symptoms (12 cases, 60%), followed by peach (10, 50%). Tomato was the most frequent among the vegetables (four cases, 20%).
Nine (45%) patients reported intolerance to a single food (seven to kiwi, one to peach, and one to strawberry).
Urticaria/angio-oedema and OAS were the most reported symptoms of food allergy, each presenting in six children (30%), equitably with anaphylaxis, defined according to the clinical criteria of Sampson.19
Sensitization to pan-allergens: skin prick tests and specific IgE performanceAllergy to more than one fruit or vegetable was observed in 11 (55%) children. Sensitization to tested pan-allergens by SPT was found in 11 children, and by sIgE in 12 children.
Patients with multiple food allergy were more likely to be sensitized to pan-allergens (assessed by SPT), than patients allergic to a single fruit or vegetable (OR=15.8).
Through the methodology of the SPT, sensitization to Pho d 2 (Profilin), Mal d 1 (PR-10 allergen), and Pru p 3 (LTP), was detected in five (25%), seven (35%) and eight (40%) cases, respectively. In eight children there were co-sensitizations between the pan-allergens: PR-10 allergens – LTP, five cases; LTP – Profilins, three cases; PR-10 allergens – Profilins, two cases, and PR-10 allergens – LTP – Profilins, one case.
By using the CAP system, four (20%) patients had positive results simultaneously to rBet v 2 and Phl p 12 (profilins), two (10%) to rBet v 1 (PR-10 allergen), and 11 (55%) to Pru p 3 (LTP). Positive sIgE to Pru p 3 ranged from 0.37 to 21.0kU/L.
Given the high number of children with pollen sensitization (16, 80.0%), no statistically significant association was demonstrated between sensitization to pollens and any of the pan-allergens tested. However, we confirmed the link between profilins sensitization and pollen allergy: all children with sensitization to profilins had sensitization to pollens, but many without sensitization to profilins also had allergy to pollens. Of the eight patients sensitized to LTP by SPT, six had positive SPT to pollens.
Using the SPT method, LTP sensitization seemed to be associated to peach allergy and vice versa (87.5% and 70% of cases, respectively), and profilins sensitization to melon (60% and 100%) and tomato (40% and 50%) allergy (Tables 2 and 3). Kiwi sensitization seemed mostly species-specific. Of the 12 children with kiwi allergy, only one had positive SPT to Pho d 2, four to Mal d 1, and three to Pru p 3, which are a minority.
LTP sensitization was not linked with severe reactions, i.e. anaphylactic reactions (one out of eight children), nor did it occur for profilin- or PR-10 allergens-sensitization.
The majority of the anaphylactic reactions occurred in children with single allergy to kiwi (four cases, 57.1%).
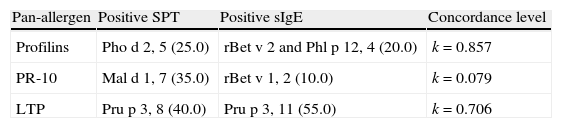
The concordance of positive results between SPT extracts and sIgE to the corresponding pan-allergens was evaluated. There was a high concordance for profilins (k=0.857) and LTP (k=0.706), while for PR-10 allergens concordance it was absent (k=0.079) (Table 4).
Concordance of SPT and sIgE with single-molecular allergen components to the corresponding pan-allergens.
| Pan-allergen | Positive SPT | Positive sIgE | Concordance level |
| Profilins | Pho d 2, 5 (25.0) | rBet v 2 and Phl p 12, 4 (20.0) | k=0.857 |
| PR-10 | Mal d 1, 7 (35.0) | rBet v 1, 2 (10.0) | k=0.079 |
| LTP | Pru p 3, 8 (40.0) | Pru p 3, 11 (55.0) | k=0.706 |
Data are presented as N (%). LTP – Lipid Transfer Proteins; PR-10 – Pathogenesis-Related protein family 10; sIgE – specific IgE; SPT – skin prick tests.
A field in which molecular diagnosis is proving to be crucial is in food allergy. In children, this issue is particularly important considering that the identification of species-specific allergen components singles out the primary sensitizer that, eventually, may vanish over time.
In patients allergic to fruits and vegetables, the panel of cross-reactive allergens – profilins, PR-10 allergens and LTP – has been applied in experimental settings, with proven clinical utility, and provided the rationale that supported our study.5,9,10,20 We aimed to evaluate the diagnostic usefulness of SPT with single-molecular allergen components in children with allergy to fruits and vegetables. Simultaneously, we intended to validate the SPT results via comparison with the corresponding sIgE, in a molecular approach. For this purpose, we selected a population of patients with documented food allergy.
The most frequent clinical presentations of allergy in our patients were urticaria/angio-oedema, OAS and anaphylaxis in six (33.3%) children for each manifestation. These results are in accordance with the literature.21 Atopic dermatitis is commonly associated with food allergy in children, but rarely in adults.22 Three children had just this one manifestation. Immediate gastrointestinal hypersensitivity reactions as respiratory symptoms are rarely isolated, and most often accompany allergic symptoms in other target organs. Nevertheless, rhinoconjunctivitis and/or bronchospasm following inhalation of food dusts or vapours are not rare in food-allergic patients and have been associated with some foods, including legumes.23
Fresh fruits are commonly involved in food-allergic reactions in children, with the Rosaceae family fruits dominating, and vegetables being rarer. According to the European Community Respiratory Health Survey, sensitization to fruits was dominated by peach (5.4%), apple (4.2%) and kiwi (3.6%) followed by banana (2.5%) and melon (1.6%). Among vegetables, celeriac, carrot and tomato were the most frequent, with an overall prevalence of sensitization of 3.6%, 3.5% and 3.3%, respectively.24 Our study corroborated this evidence, with minor differences, which may be attributed to the smaller sample. Nevertheless, it is interesting to point out that peach elicited 3.3 times more allergy than apple, although apple was consumed more often than peach.
A close relationship between fruit and vegetable allergy and sensitization to pan-allergens was observed. Sensitization detected by SPT occurred in 11 (55%) children, and by sIgE in 12 (60%), mainly to Pru p 3 LTP, in both methods (40%, and 55%, respectively), with high concordance levels (k=0.706). Nevertheless, the best value of concordance was found for profilins, comparing SPT to Pho d 2 with sIgE to rBet v 2 and Phl p 12 (k=0.857).
The SPT performance for PR-10 allergens, explored by Mal d 1 when compared to sIgE to rBet v 1 was poor (k=0.079), apparently with many false positives in SPT. The information extracted from the literature points to a low prevalence of sensitization to PR-10 allergens in countries of southern Europe.4 Since such a large number of sensitizations were not confirmed by sIgE, we hypothesize that the preparation of the Mal d 1 extract might not have been done appropriately and contains other allergenic proteins that must also be taken into account (e.g. the LTP Mal d 3). In this sense we evaluated the correlation between the prick test results of the apple and peach extracts, detecting a moderate correlation between them (k=0.420). Therefore, the authors point out that the spiked apple extract presumably cannot be considered an appropriate marker for sensitization to PR-10 allergens.
Another question that remains a matter of controversy is whether allergen-specific IgE antibody levels correlates with cutaneous sensitivity. Niederberger et al. demonstrated considerable discrepancies between antibody levels and biological sensitivity on a molecular level, using the same allergens.25 These discrepancies could be even greater when the allergens employed in the two methods are different.
Reactivity to birch pollen might be an indirect indicator of PR-10 sensitization. Four children had SPT positive to the birch pollen extract, and of these, only two had positive sIgE to rBet v 1. In Southern Europe, where birch trees are uncommon or absent, a positive test to birch pollen often reflects sensitization to PR-10 allergens present in other trees closely related to birch (e.g. alder, hazel, hornbeam, beech and chestnut) or sensitization to other pollen allergens such as profilins (Bet v 2 homologues) in grass and weed. Thus, the presence of birch pollen sensitization also does not seem to be the most correct way to detect sensitization to PR-10 allergens.
The main foods implicated in PR-10 allergy are apple and other fruits of the Rosaceae family (including pear, peach, cherry and plum), hazelnut, vegetables of the Apiaceae family (including carrot and celery), kiwi, and/or foods of the Fabaceae family (e.g. soybean and peanut).14 In our study, apple allergy was not linked to any one pan-allergen sensitization. Furthermore, it was not possible to establish a relationship between some kind of fruit or vegetable allergy and sensitization to PR-10 allergens.
The symptoms are usually mild and restricted to OAS cases. However, severe allergic reactions have been ascribed from fresh celery, carrot and soybean, even if these foods are processed to a certain degree. Interestingly, several studies have shown that birch pollen-related foods may induce worsening of atopic dermatitis a few days after exposure to fresh or cooked foods.28,29 Our children who had pruritus/eczema as the only manifestation of food allergy were not predominantly sensitized to PR-10 allergens.
Profilins are one of the main causes of cross-sensitization between pollen and plant-derived foods, even between botanically unrelated species.4 In the Mediterranean area, in patients allergic to the Rosaceae family fruits, the frequency of sensitization to profilins is approximately 40%, although it can rise to 75% in patients with associated pollinosis, caused by grass, weed, and trees.26 Our study confirmed the link between profilins sensitization and pollen allergy. The most frequently offending foods are melon, watermelon, tomato, banana and citrus fruits, being considered as clinical markers of profilins hypersensitivity, once latex allergy has been ruled out.27 Regarding in vitro diagnostic markers there were no differences between sIgE to birch and grass pollen profilin, signalling that the two tests do not perform differently, but with consistently higher values for rBet v 2.
The clinical relevance of profilins as a food allergen is still debated. More than one half (57%) of profilin reactors can have food allergy,26 expressed only by the typical OAS, which is consistent with the known heat and pepsin-sensitivity of this allergen. In a small minority of patients, however, profilins can cause severe reactions. Nevertheless, since most of these patients were also sensitized to other allergens, the role played by profilin in such reactions remains to be established. Our patients sensitized to profilins presented mostly mild reactions, with only one mono-sensitized showing a severe reaction – anaphylaxis with tomato, but in which the latex allergy may be a differential diagnosis. Another one with a severe reaction revealed sensitization to LTP in addition to profilins. Once again, the authors stress the drawback in this study concerning the establishment of a relationship between a sensitization profile and severity grade of the clinical manifestations, considering the small and heterogeneous sample, and therefore, no one statistical analysis was done.
LTP have been identified as allergens in a range of fruits, vegetables and even nuts. Allergy to peach (Prunus persica) is the “trademark” of LTP hypersensitivity, being the most prevalent plant food allergy in the Mediterranean area, in the absence of pollen allergy.4 Among peach allergic patients, 80% are sensitized to LTP, which is mainly found in the peel of the fruit.30 In our study, from eight children sensitized to LTP by SPT, seven (87.5%) had allergy to peach, supporting this association. The mugwort LTP Art v 3 has been shown to display some limited cross-reactivity to the Rosaceae fruit LTP and may play a role in pollen – food syndromes associated with weed pollen,31 which was not observed in our study. Cross-reactivity between Parietaria LTP Par j 2 and other fruit's and vegetables’ LTP is very limited.32
There was no association between severe reactions and LTP sensitization. LTP are thermostable and resistant to pepsin digestion, which can make them potent food allergens, but can also be manifested only by isolated OAS. Some patients allergic to the LTP of Rosaceae fruits (around 1/3) may tolerate the pulp of these fruits, and reactions can only be observed when exercise, or other co-factors are associated.4,20 Asero et al. in a study conducted in 2008 likewise found that most of the patients sensitized to stable vegetable food allergens reported OAS.12
One of the main objectives of detecting LTP sensitization is the possibility of carrying out a tailored immunotherapy, considering that there is a prevalent, persistent and potentially severe allergy. Some preliminary data on peach6,33 seem promising.
Allergy to peach and apple was amply discussed and enclosed in our patients, but another plant-derived food that is causing an increasing number of allergies is kiwi, as substantiated by our study. In fact, kiwi allergy is not a homogeneous disorder, with symptoms ranging from mild local reactions up to severe systemic symptoms. It appears that when kiwi allergy is not associated with pollen allergy, these patients have the highest risk of systemic reactions.34 Kiwi allergy can either be associated with sensitization to PR-10 allergens, profilins and latex.35,36 In addition, Act d 10, the kiwi LTP, and Act d 1, significantly related to kiwi mono-sensitization, were identified as relevant kiwi allergens.12 Albeit unconfirmed by molecular tests, our study suggests that a majority of cases were related to species-specific sensitization, with 4 cases of severe reactions in single kiwi-allergic children.
Patient number 5 had latex allergy being the cross-reactivity phenomena the most likely cause of her food allergies. Profilins sensitization in patient number 7 can explain the latex sensitization in SPT and sIgE by standard methods, but does not exclude sensitization to other major latex allergens also involved in the cross-reactivity phenomena. Assays for example for Heb v 6.02 would be an interesting confirmatory test to perform.
Concerning vegetables, we detected four children with allergy to tomato and one to carrot, which are yet among the most prevalent in the literature.
The allergenic properties of tomato, as a result of the supposed potential to induce unspecific histamine liberation, have been mainly neglected in the past. Four tomato allergens have been accepted to date: Lyc e 1 (profilin), Lyc e 2 (β-fructofuranosidase), Lyc e 3 (LTP) and Lyc e 4 (PR-10 allergen), but were not yet applied in a CRD approach. Cross-reactivity between tomato and pollen sensitization has been observed.37 In our children, tomato allergy seemed to be linked with profilins sensitization (50% of cases).
Carrot allergy has mainly been observed in relation to a concomitant pollen allergy. Up to 50% of patients develop systemic reactions. Ballmer-Weber et al. demonstrated that 98% of Central European carrot-allergic patients were sensitized to the PR-10 allergen Dau c 1.0104, and 38% recognised the carrot profilin, Dau c 4.38 The diagnostic relevance of other discovered allergens has also not been investigated to date in a CRD study.
Our study has some limitations. The panel of plant-food allergens that we employed was incomplete, as other cross-reacting allergens – such as the thaumatin-like proteins (TLP), seed storage proteins and cross-reactive carbohydrate determinants (CCD) – might be involved. TLP have been identified as allergens in apple, cherry, bell pepper, kiwi, orange and grapes.39 Their real clinical significance remains ill-defined due to the extreme rarity of mono-sensitized patients.14 In patients sensitized to species of the Rosaceae family and/or nuts, the possibility of sensitization to storage proteins should be suspected. Sensitization to CCD epitopes may cause mild or even severe reactions in a small minority of patients, such as in the case of tomato, celery and zucchini allergy.40 Other limitations of this study include the small sample size making it difficult to reach any definitive conclusions, and the drawback inherent to the limited commercial availability of purified allergens, used for in vivo and in vitro molecular diagnosis in the field of fruit and vegetable allergy. Their preparation is generally difficult and critically dependent on several factors such as the allergenic sources, the lability of the allergens and the manufacturing process, thereby causing an inadequate allergen representation.23 Additionally, molecular diagnostic tests must be validated and reproducible.
ConclusionsPan-allergen sensitization in children with allergy to fruits and vegetables was found to be common and often multiple. The currently accepted idea – in which primary sensitization to a major food allergen induces more severe reactions than the more easily degradable allergens responsible for secondary food allergy – does not seem to account for all clinical realities, particularly regarding food-allergic children. There was no association between severe reactions and LTP sensitization, which was the most frequent pan-allergen involved. Profilins should be considered a clinically relevant food allergen. The introduction of routine SPT to pan-allergens can be a simple and feasible way of improving diagnostic and therapeutic efficacy. Patients should be tested by an adequate panel of allergenic molecules, mainly considering their clinical relevance, and variations in geographic area.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data. All parents or legal guardians have received sufficient information and provided informed consent regarding each child's participation in the study.
Right to privacy and informed consentThe authors have obtained the informed consent of the parents or legal guardians of the children mentioned in the article. The author for correspondence is in possession of this document.
FundingThe present authors would like to thank to ALK-Abelló-Spain Laboratories for the extracts to perform the skin prick tests with pan-allergens.
Conflicts of interestThe authors have no conflict of interest to declare.