Exhaled nitric oxide (FeNO) has been introduced in the diagnosis and control of asthma. Atopy related diseases are a common comorbidity in asthma, but in these cases the FeNO values and their relevance have not been clearly defined. In this study we compared the differences in FeNO levels in various atopic conditions.
MethodsA prospective study was performed comparing online FeNO in six groups of patients (non-atopic control, asymptomatic atopic, non-active rhinitis, active rhinitis, asthma, asthma with rhinitis).
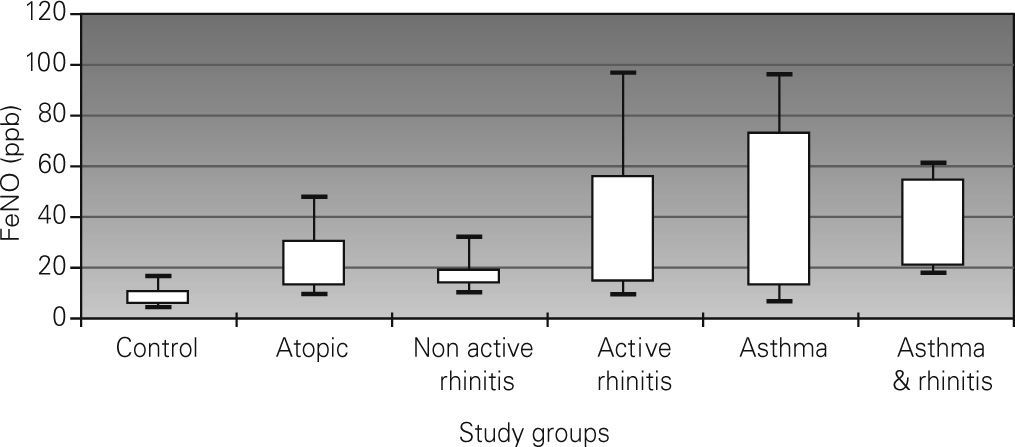
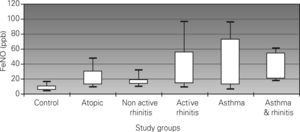
Results90 children (15 per group) assisted in an outpatient hospital clinic were enrolled. FeNO levels (ppb) were: 7.9 (non-atopic control), 19 (asymptomatic atopic), 16.6 (non-active rhinitis), 26.3 (active rhinitis), 31 (asthma), and 35 (asthma and rhinitis). All groups presented higher levels of FeNO than the non-atopic control group (P<0.001). Both asthma groups had higher levels than the rest of the groups (P<0.05), except for the active rhinitis group. Dispersion of FeNO levels was wide in the study sample. No correlation was found between FeNO and FEV1. A weak correlation was seen with age (r=0.28)
ConclusionsAtopy, rhinitis and its exacerbations can be confounders in the interpretation of FeNO levels in asthmatic children.
Exhaled nitric oxide (FeNO) has proved to be a marker of inflammation in the airways. Initially used for investigation purposes, FeNO has recently been introduced into clinical practice. Although believed to be highly specific of pulmonary inflammation, recent reports on FeNO have shown increased levels in non-asthmatic patients with other atopy related diseases such as rhinitis1,2 or atopic eczema3–5. The increase of FeNO in non-asthmatic individuals suggests that FeNO is not only a reflection of pulmonary pathology but also of other atopic conditions. The evaluation of the degree of control of asthma inflammation is sometimes difficult, especially in children. For this reason, physicians have introduced FeNO with the aim of having an objective parameter which could account for the extent of asthma control. Unfortunately, the fact that FeNO may be modified by other factors can lead to errors and raises concern on the interpretation of increased levels of FeNO in asthmatic children. The high prevalence of atopy related diseases amongst asthmatic patients makes it important to define the influence of these atopic conditions on FeNO.
Most studies that analyse FeNO and atopy in children have been performed in samples obtained from birth cohort studies3, ISAAC study6,7 or schoolchildren2,8. Although they could have the advantage of avoiding a selection bias, these samples are not representative of the patients seen in paediatric allergy or pneumology departments. On the other hand, diagnoses of atopic eczema, allergic rhinitis or asthma are sometimes based on questionnaires which are less accurate than a physician's evaluation. Some of these reports have analysed the influence of allergic rhinitis but have not considered the impact of a recent exacerbation of rhinitis symptoms on FeNO1,2,6,7,9.
In this study we try to determine the FeNO levels in several common atopic conditions with a sample that was representative of the children evaluated in clinical practice.
MATERIAL AND METHODSStudy population and designThis study was performed at Elche General University Hospital, a tertiary care hospital in the city of Elche (Alicante, Spain), with a catchment area of 330,000 inhabitants and 57,000 children younger than 16years of age. The city is geographically situated on the eastern coast of Spain, 10km from the sea.
A prospective study was conducted to compare FeNO levels in 6 different groups of children younger than 16years. These groups were the following: 1) asymptomatic children without allergen sensitisation (control group); 2) asymptomatic children with allergen sensitisation (asymptomatic atopic group); 3) patients with allergic rhinitis without any exacerbation in the last month (non-active rhinitis group); 4) patients with rhinitis with one exacerbation in the last month (active rhinitis group); 5) patients with allergic asthma without rhinitis (asthma group); 6) patients with allergic asthma with rhinitis (asthma & rhinitis group). All consecutive patients that were attended in our department who fulfilled the above inclusion criteria were invited to participate, up to a total of 15 patients per group. Two children were excluded after being unable to achieve a correct FeNO reading and seven patients declined to enrol.
All the children had a thorough anamnesis, were systematically tested with skin prick tests (SPT), underwent pulmonary function test and exhaled nitric oxide determination.
Skin prick testsPatients were tested with a panel of the most common food and aero-allergens (ALK-Abello): Dermatophagoides pteronysinus, Dermatophagoides farinae, Blatella germanica, cat dander, dog dander, rabbit dander, horse dander, Alternaría, Arpergillus, Olea europaea, Artemisia, Parietaria judaica, mixed grasses (Dactiylis, Lolium, Festuca, Poa, Phelum and Avena), cow's milk, egg white and blue fish, almond, peanut, orange and peach. Histamine (10mg/ml) and saline solution (0.9 %) were used as positive and negative controls, respectively. If other allergen sensitisation was suspected it was added to the allergen panel. SPT were performed by an expert nurse with ALK-Abello (Madrid, Spain) lancets. A child was considered to be sensitised to a specific allergen if a wheal diameter measuring 3mm or more (after subtraction of the control value) was observed 15 minutes after the prick.
Measurement of pulmonary function and exhaled nitric oxideFeNO was measured online according to the recommendations of the ERS/ATS standard10 using a portable nitric oxide analyser (NIOX-MINO; Aerocrine; Sweden)11–13. After a period of training and demonstration performed by one of the members of the department, children underwent FeNO measurements.
Spirometry was performed with Datospir 120 (Sibelmed, Spain), according to the ATS/ERS recommendations14,15.
Diagnostic criteriaAsthma was defined as a department doctor's diagnosis. Current allergic asthma diagnosis was made if asthma symptoms were present within the last twelve months and at least one skin allergen sensitisation was clinically related to these. Recent asthma crisis was defined as an increase in asthma symptoms within the last month which required the use of relief medication for at least 24 hours. Rhinoconjuntivitis diagnosis required frequent or seasonal symptoms with two or more of the following: itchy nose, rhinorrhoea, sneezing, sneezing fit, nasal blockage, red and itchy eyes. A rhinoconjunctivitis exacerbation was defined as an increase in basal symptoms during the previous month. Atopic dermatitis was diagnosed following Hanifin and Rajka diagnostic criteria16. Family history of atopy was defined as one or more of the following: physician diagnosed asthma, suggestive history of allergic rhinitis/conjunctivitis, physician diagnosed allergic eczema or any other confirmed IgE mediated allergies in at least one parent or sibling.
Statistical analysesData were processed using a Microsoft office Excel database. Statistical analyses were performed using SPSS 10.0. FeNO values were log transformed for analysis and transformed back again into ppb. Comparisons between groups were performed using student'st-test. FeNO values were given in mean and 95 % confidence interval range or medians and interquartile ranges. The distribution of the remaining variables was given in mean, 95 % confidence or range. Correlation between variables was tested using Pearson's correlation test.
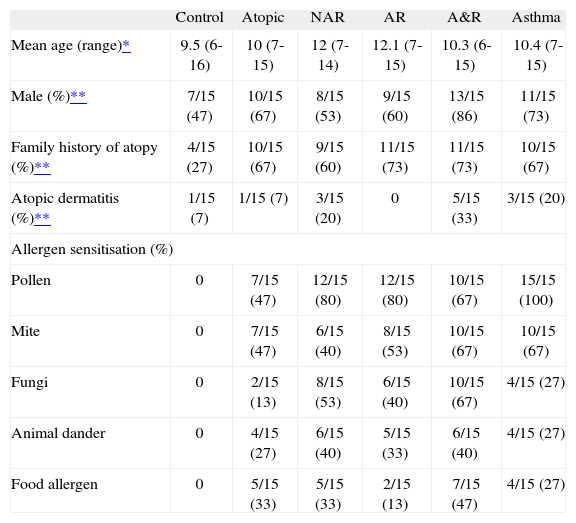
RESULTSCharacteristics of the sampleFrom October to December 2007, a total of 90 children less than 16years of age were studied (15 children per group). Mean age was 10.8years (range 6-15years). Fifty eight out of ninety (64.4 %) were male. Family history of atopy was present in 55/90 (61.1 %) children. The most common sensitisations were to pollen (56/75, 74.7 %) and mite (41/75, 54.7 %). Food allergen, animal dander and fungi sensitisation were present in 23/75 (30.7 %), 26/75 (34.7 %) and 30/75 (40 %), respectively. All sample groups were homogeneous for age, sex, atopic eczema and family history of atopy (FHA) except for the control group in which there was a female predominance (47 % male, p = 0.2) and a smaller prevalence of FHA (4/15 vs. 51/75, p < 0.001). General characteristics by groups are shown in Table Table I. Children in the asthma and asthma & rhinitis groups had a recent asthma crisis in 4/15 (26.7 %) and 7/15 (46.7 %) cases, respectively. FEV1 values were higher in the control group compared to the other groups separately, but the differences did not achieve statistical significance. No differences were observed in other spirometric indices (Table Table II).
Main characteristics of the sample groups
| Control | Atopic | NAR | AR | A&R | Asthma | |
| Mean age (range)* | 9.5 (6-16) | 10 (7-15) | 12 (7-14) | 12.1 (7-15) | 10.3 (6-15) | 10.4 (7-15) |
| Male (%)** | 7/15 (47) | 10/15 (67) | 8/15 (53) | 9/15 (60) | 13/15 (86) | 11/15 (73) |
| Family history of atopy (%)** | 4/15 (27) | 10/15 (67) | 9/15 (60) | 11/15 (73) | 11/15 (73) | 10/15 (67) |
| Atopic dermatitis (%)** | 1/15 (7) | 1/15 (7) | 3/15 (20) | 0 | 5/15 (33) | 3/15 (20) |
| Allergen sensitisation (%) | ||||||
| Pollen | 0 | 7/15 (47) | 12/15 (80) | 12/15 (80) | 10/15 (67) | 15/15 (100) |
| Mite | 0 | 7/15 (47) | 6/15 (40) | 8/15 (53) | 10/15 (67) | 10/15 (67) |
| Fungi | 0 | 2/15 (13) | 8/15 (53) | 6/15 (40) | 10/15 (67) | 4/15 (27) |
| Animal dander | 0 | 4/15 (27) | 6/15 (40) | 5/15 (33) | 6/15 (40) | 4/15 (27) |
| Food allergen | 0 | 5/15 (33) | 5/15 (33) | 2/15 (13) | 7/15 (47) | 4/15 (27) |
NAR: non-active rhinitis; AR: active rhinitis; A&R: asthma and rhinitis.
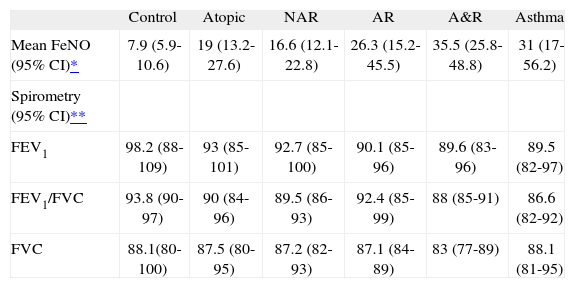
Differences in exhaled nitric oxide levels and the main spirometric indices between the study groups
| Control | Atopic | NAR | AR | A&R | Asthma | |
| Mean FeNO (95% CI)* | 7.9 (5.9-10.6) | 19 (13.2-27.6) | 16.6 (12.1-22.8) | 26.3 (15.2-45.5) | 35.5 (25.8-48.8) | 31 (17-56.2) |
| Spirometry (95% CI)** | ||||||
| FEV1 | 98.2 (88-109) | 93 (85-101) | 92.7 (85-100) | 90.1 (85-96) | 89.6 (83-96) | 89.5 (82-97) |
| FEV1/FVC | 93.8 (90-97) | 90 (84-96) | 89.5 (86-93) | 92.4 (85-99) | 88 (85-91) | 86.6 (82-92) |
| FVC | 88.1(80-100) | 87.5 (80-95) | 87.2 (82-93) | 87.1 (84-89) | 83 (77-89) | 88.1 (81-95) |
NAR: non-active rhinitis; AR: active rhinitis; A&R: asthma and rhinitis.
A normal distribution was observed for FeNO values in the six study groups after using logarithmic transformation. Mean values of FeNO are shown in Table Table II. Statistically significant differences were observed between the FeNO mean values of the control group and the rest of the groups (p < 0.001). Significant differences in FeNO were also observed between the groups of asthma and asthma & rhinitis with the groups of asymptomatic atopic and non-active rhinitis (p < 0.05). No statistically significant differences were observed between the groups of asthma, asthma & rhinitis and active rhinitis (31 and 35.5ppb vs. 26.3ppb, respectively). The dispersion of FeNO values was wide in all the groups (excluding the control group) but it was more relevant in the groups of asthma (interquartile range: 12.9-73ppb) and active rhinitis (interquartile range: 14-56.4ppb). Figure 1 shows the differences between groups.
FeNO levels were higher (p < 0.05) in males (mean 24.1ppb; CI95 %: 23.4-24.8ppb) than in females (15.2ppb; CI95 %:14.4-16ppb); although statistically significant (p < 0.01) correlation between FeNO levels and age was weak (r = 0.28, CI95 %, 0.08-0.46)
Correlation between FeNO and pulmonary functionNo correlation was observed between FeNO and FEV1 when analysed per groups or in the complete sample (r = –0.18; CI95 %:–0.37 to 0.28). Correlation was not found either between other spirometric indices (FVC or FEV1/FVC) and FeNO.
DISCUSSIONAll the groups in our study including that of atopic asymptomatic children had higher FeNO levels than the asymptomatic controls. Interestingly, the group of atopic children did not differ from the group of patients with non-active rhinitis. Several studies have suggested that allergen sensitisation could alone elevate FeNO levels17,18. In our study, we have observed that the exacerbation of rhinitis symptoms resulted in an increase in FeNO levels. Alhough some studies have reported that rhinitis is an independent factor for FeNO17, few studies have analysed the FeNO levels in exacerbations of rhinitis symptoms. In a recent report, van Asch et al3 did not find differences between patients with rhinitis or asthma. In our study, we have not found differences between patients with asthma and active allergic rhinitis. However, we have observed statistically significant differences between asthma and non-active allergic rhinitis.
We have analysed the correlation between FeNO levels and different variables such as sex, age, and spirometric indices. As observed in previous studies in adults17,19 and children3,7,8, we have also found significantly higher FeNO levels in male than in female children. With regard to age, its correlation with FeNO levels was weak. Although this correlation has been previously reported2, the factors involved are not known. The fact that FeNO increases mainly during childhood suggests that it could be related to an increase in the airway surface area or the increase in allergen sensitisation that occurs during childhood. In accordance to literature7,19,20 we have not observed any correlation between FeNO and the most common spirometric indices. Interestingly, we did not find statistically significant differences in spirometric indices between the study groups, but this could be related to the limitation of the sample size.
The degree of dispersion in FeNO levels was very high except for the asymptomatic control group, and this variability probably reflects the degree of inflammation in the airway. An important FeNO variability was also observed in the group of healthy atopic patients. This observation suggests that there could be increased FeNO levels and/or pulmonary inflammation without clinical symptoms or spirometric changes. Whether high levels of FeNO are due to a subclinical increase in pulmonary inflammation or not, the high degree of dispersion in FeNO levels can cause confusion in the clinical practice.
In this paper, we have reported mean FeNO values in different groups of patients representing those controlled in a paediatric pneumology and/or allergy department. As we have observed, FeNO values can be affected by multiple factors and not only by asthma. Therefore, FeNO should be regarded as a complementary tool which combined with others can be useful in the diagnosis of asthma and its control, predicting patients who would benefit from inhaled corticosteroid use21. On the other hand, FeNO levels used in asthma control can differ from those expected according to their clinical symptoms or pulmonary function tests. In these cases it is recommendable to consider the effect of other atopic conditions before modifying the asthma treatment.
In conclusion, our study reports FeNO values using NIOX-MINO in patients with common atopic conditions found in clinical practice. We have observed that FeNO levels in these atopic conditions overlap the levels observed in patients with asthma. Therefore, the coexistence of atopic conditions should be considered when FeNO is used for the control or diagnosis of asthma.