Immunoglobulin E-mediated allergy to cow's milk protein (CMP) tends to subside over years of follow-up. The gold standard for detecting such allergy has been the oral challenge test. The development of some other test for determining the correct timing of the oral challenge test would avoid unnecessary patient discomfort.
The aim of this study was to determine whether monitoring cow's milk (CM) specific IgE levels over time can be used as a predictor for determining when patients develop clinical tolerance.
MethodsA prospective 4-year follow-up study was made of 170 patients with IgE-mediated allergy to CMP, involving periodic evaluations (12, 18, 24, 36 and 48 months) with the determination of casein and CM specific IgE on each visit, along with CM challenge testing. ROC curves were used to analyse the sensitivity, specificity and predictive values of the casein and CM specific IgE levels versus the challenge test outcomes at the different moments of follow-up.
ResultsIn the course of follow-up, 140 infants (82 %) became tolerant. Specific IgE levels to CM: 2.58, 2.5, 2.7, 2.26, 5kUA/l and to casein: 0.97, 1.22, 3, 2.39, 2.73kUA/l, respectively, predicted clinical reactivity (greatest diagnostic efficiency values) at the different analysed moments of follow-up (12, 18, 24, 36 and 48 months).
ConclusionsQuantification of CMP specific IgE is a useful test for diagnosing symptomatic allergy to CM in the paediatric population, and could eliminate the need to perform oral challenges tests in a significant number of children.
Cow's milk (CM) allergy affects about 2.5 % of all infants in the first year of life1,2, with IgE-mediated reactions accounting for about 60 % of milk allergy-related disorders (1.5 % of all infants). Although most infants with CM allergy outgrow their sensitivity by the fourth year of life3, 14-15 % of infants with IgE-mediated CM allergy retain sensitivity into the second decade4,5.
Allergy to cow's milk proteins (CMP) demands a strict elimination diet and hydrolysed substitutes or soy formulas as appropriate treatment for children. Clinical reactivity may persist for many years, with the consequent risk of reaction secondary to accidental exposure. Some patients may suffer severe and even life-threatening anaphylactic reactions due to small inadvertent exposure in the form of allergen occult in food. On the other hand, dietary restriction has an impact upon patient and family quality of life, due to its social repercussions (school lunchroom, parties, etc.), with the added risk of nutritional disorders. In turn, the fear of a new reaction produces patient and family anxiety, resulting in patient overprotection. Thus, the earliest possible detection that the allergy has been overcome is very important for both patient and family.
Although challenge tests are time consuming and risky, they are needed to determine whether children have or have not outgrown their CM allergy. Efforts have been made in recent years to find appropriate methods allowing us to avoid the performance of challenge tests with a high probability of proving positive, and to carry out those challenge tests with a high probability of proving negative. The assay of food-specific IgE concentration with the CAP system (Pharmacia) is considered to be an appropriate method both for long-term prognosis3 and for predicting clinical reactivity at a specific moment6–8. The application of this method could eliminate the need to perform challenge tests in a considerable number of children.
The present study analyses specific IgE levels to CM and casein at different moments of follow-up in infants diagnosed with IgE-mediated allergy to CMP in the first year of life, with the aim of determining cut-off points to help in differentiating between tolerant and non-tolerant children during follow-up.
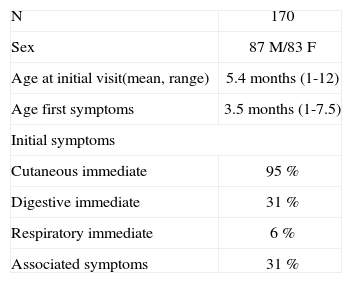
MATERIALS AND METHODSStudy populationA total of 170 infants (87 males and 83 females) diagnosed with IgE-mediated allergy to CMP were enrolled in this prospective follow-up study. Briefly, the patients were diagnosed with CMP allergy on the grounds of a compatible clinical picture of immediate hypersensitivity reaction to CM confirmed by prick, CAP and open challenge testing to CM. The challenge test was not carried out in 8 infants due to contraindication because of the severe symptoms of the patients, and (in accordance with previous studies9) in 73 patients who met all the following criteria: urticaria and/or angio-oedema; the appearance of symptoms in the first 60 minutes after intake; positive skin tests (≥ 3mm) and specific IgE to CM ≥ 3 kU/l; and less than three months since the last clinical reaction. Table I reports the patients' characteristics.
Once they were diagnosed, the patients were placed on a CMP elimination diet, and periodic reassessments were carried out at 12 and 18months and 2, 3 and 4years of age until tolerance was achieved, or until the last reassessment in which tolerance had not been outgrown.
On the occasion of each reassessment, we questioned the parents about possible reactions as a result of accidental ingestion of CMP, and new skin tests to CM and its proteins, as well as specific IgE determination to these allergens were performed. In addition, an open challenge test was carried out to determine whether tolerance had been achieved, or whether the allergy still persisted. At each follow-up visit, the challenge test was not performed if the child had experienced some allergic reaction clearly related to CMP ingestion within the previous three months.
Skin prick testsSkin prick tests were performed in all patients with glycerinated food extract: whole CM extract (5mg/ml) and with isolated CMP: alfa-lactalbumin (5mg/ml), beta-lactoglobulin (5mg/ml), and casein (10mg/ml)(Laboratorios Leti CBF, Barcelona, Spain). Glycerosaline solution and histamine dihydrochloride at a concentration of 1 % were applied by means of the prick technique as negative and positive controls. A net wheal diameter 3mm larger than that produced by the negative control was considered positive.
Laboratory studíesSerum samples from all patients were analysed for total serum IgE and specific IgE antibodies to milk, alfa-lactalbumin, beta-lactoglobulin and casein by using the CAP system FEIA (Pharmacia Diagnostics, Uppsala, Sweden). The test was considered positive when a result of 0.35 kUA/l was obtained.
Clínícal reactívíty evaluatíonOpen controlled challenge tests with CM were performed in the allergy unit of the hospital, where appropriate medication and resuscitation equipment were directly available. Informed consent was previously obtained from the parents. All challenge tests were carried out under the control of an allergist. The challenge test was performed in accordance with the following previously established protocol9. Two regimens freely chosen by the investigators were used: Regimen A – first day: 2ml, 5ml, 10ml; second day: 25ml, 50ml; third day: 100ml and the last dose to complete the quantity equivalent to one normal feed in accordance with the age of the child, administered at 60-minute intervals. Regimen B – in a single day, successive doses of 2ml, 5ml, 10ml, 25ml, 50ml and 120ml were given at 30-minute intervals. The challenge was considered to be positive when there were skin (urticaria, angio-oedema or erythematous rash) and/or gastrointestinal (vomiting or diarrhoea) and/or respiratory manifestations (rhinoconjunctivitis or bronchospasm) in the two hours after food intake. Clearly positive transgressions within the three months preceding each follow-up visit were assessed in the same way.
Data analysísAt each reassessment, calculations were made of the sensitivity (SE), specificity (SP), positive predictive value (PPV), negative predictive value (NPV) and efficiency (Ef) of CM specific IgE and casein IgE related to clinical reactivity. The cut-off levels of specific IgE for CM and casein were determined by analysis with the receiver-operating characteristic (ROC) curve. The analysis was performed using Graphroc software (Veli Kairisto, Turku University, Finland). Differences in the median of specific IgE among allergic and tolerant subjects were analysed with the Mann–Whitney U-test using the SPSS version 12 statistical package. In order to calculate the specific IgE medians, a value of 0.34 kUA/l was assigned to all values below 0.35 kUA/l (the lowest limit of detection when using this technique), whereas 101 kUA/l was assigned to all values above 100 kUA/l (the highest limit of detection of this technique).
RESULTSIn the course of four years of follow-up, a total of 407 challenge tests were performed in the 170 patients: 181 with regimen A and 221 with regimen B. After this period of time tolerance was gained by 140/170 patients (82 %). Percentage tolerance in the course of the study was 58/170 (34 %) after 12months, 34/170 (20 %) after 18months, 26/170 (15.3 %) after 24months, 16/170 (9.4 %) after 3years, and 6/170 (3.5 %) after 4years.
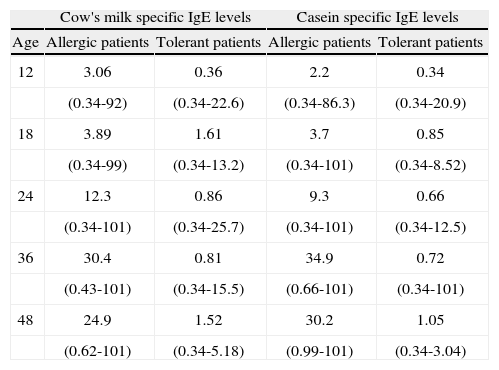
Table II reports the median specific IgE values for CM and casein in tolerant and non-tolerant patients for the different ages considered. At all ages statistically significant differences were observed between the specific IgE values of both groups (p < 0.001). Median specific IgE was seen to increase in the non-tolerant group with age up to evaluation at three years – with a tendency to decrease from that age onwards. No such behaviour was observed among the patients with tolerance.
Cow's mílk and caseín specífíc IgE levels (medían and range KU/L) ín patients wíth persístent (n = 30) versus tolerant CM allergy (n = 140)
| Cow's milk specific IgE levels | Casein specific IgE levels | |||
| Age | Allergic patients | Tolerant patients | Allergic patients | Tolerant patients |
| 12 | 3.06 | 0.36 | 2.2 | 0.34 |
| (0.34-92) | (0.34-22.6) | (0.34-86.3) | (0.34-20.9) | |
| 18 | 3.89 | 1.61 | 3.7 | 0.85 |
| (0.34-99) | (0.34-13.2) | (0.34-101) | (0.34-8.52) | |
| 24 | 12.3 | 0.86 | 9.3 | 0.66 |
| (0.34-101) | (0.34-25.7) | (0.34-101) | (0.34-12.5) | |
| 36 | 30.4 | 0.81 | 34.9 | 0.72 |
| (0.43-101) | (0.34-15.5) | (0.66-101) | (0.34-101) | |
| 48 | 24.9 | 1.52 | 30.2 | 1.05 |
| (0.62-101) | (0.34-5.18) | (0.99-101) | (0.34-3.04) | |
Age: months.
Allergic patients: patients with persistent CMA.
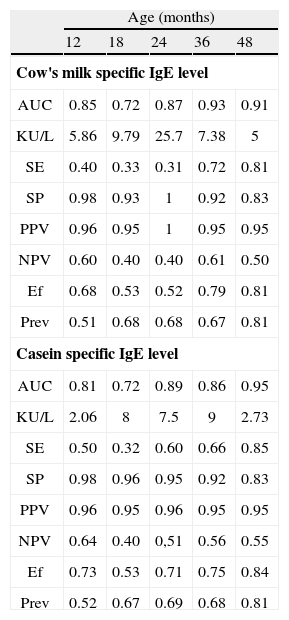
The ROC curves allow us to analyse the sensitivity and specificity of the specific IgE levels for CM and casein in relation to the results of the challenge tests. Table III show the areas under the ROC curves at different moments of follow-up. Very good values were obtained for CM at 36 and 48months, and at 48months for casein. Table III in turn reports the performance characteristics of the specific IgE levels for CM and casein, with a predictive value of 95 % at the different moments analysed. As can be seen, these values offer high specificity, though the sensitivity is very low for the first moment of follow-up analysed(corresponding to the visits after 12, 18 and 24months). Greater efficiency was seen at evaluation after 36 and 48months for CM, and after 48months for casein.
Performance characteristics of cow's milk and casein specific IgE levels (VPP > 95 %) at the follow-up
| Age (months) | |||||
| 12 | 18 | 24 | 36 | 48 | |
| Cow's milk specific IgE level | |||||
| AUC | 0.85 | 0.72 | 0.87 | 0.93 | 0.91 |
| KU/L | 5.86 | 9.79 | 25.7 | 7.38 | 5 |
| SE | 0.40 | 0.33 | 0.31 | 0.72 | 0.81 |
| SP | 0.98 | 0.93 | 1 | 0.92 | 0.83 |
| PPV | 0.96 | 0.95 | 1 | 0.95 | 0.95 |
| NPV | 0.60 | 0.40 | 0.40 | 0.61 | 0.50 |
| Ef | 0.68 | 0.53 | 0.52 | 0.79 | 0.81 |
| Prev | 0.51 | 0.68 | 0.68 | 0.67 | 0.81 |
| Casein specific IgE level | |||||
| AUC | 0.81 | 0.72 | 0.89 | 0.86 | 0.95 |
| KU/L | 2.06 | 8 | 7.5 | 9 | 2.73 |
| SE | 0.50 | 0.32 | 0.60 | 0.66 | 0.85 |
| SP | 0.98 | 0.96 | 0.95 | 0.92 | 0.83 |
| PPV | 0.96 | 0.95 | 0.96 | 0.95 | 0.95 |
| NPV | 0.64 | 0.40 | 0,51 | 0.56 | 0.55 |
| Ef | 0.73 | 0.53 | 0.71 | 0.75 | 0.84 |
| Prev | 0.52 | 0.67 | 0.69 | 0.68 | 0.81 |
AUC: area under curve; SE: sensitivity; SP: specificity; PPV: positive predictive value; NPV: negative predictive value; Ef: Efficiency; Prev: Prevalence.
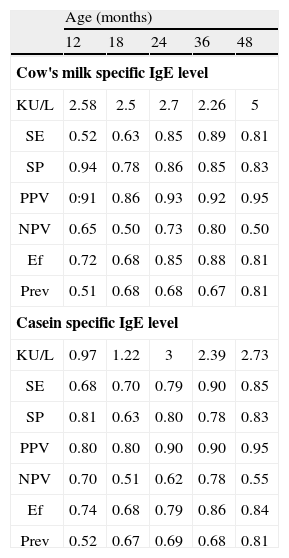
Analysis of the ROC curves allows us to identify the optimal decision points, i.e., those points where sensitivity and specificity are more balanced. Table IV expresses these cut-off values for CM and casein at the different evolutive timepoints. As can be seen, specificity decreases while sensitivity increases.
Performance characteristics of the optimal decision point of cow's milk and casein specific IgE level in the follow-up
| Age (months) | |||||
| 12 | 18 | 24 | 36 | 48 | |
| Cow's milk specific IgE level | |||||
| KU/L | 2.58 | 2.5 | 2.7 | 2.26 | 5 |
| SE | 0.52 | 0.63 | 0.85 | 0.89 | 0.81 |
| SP | 0.94 | 0.78 | 0.86 | 0.85 | 0.83 |
| PPV | 0:91 | 0.86 | 0.93 | 0.92 | 0.95 |
| NPV | 0.65 | 0.50 | 0.73 | 0.80 | 0.50 |
| Ef | 0.72 | 0.68 | 0.85 | 0.88 | 0.81 |
| Prev | 0.51 | 0.68 | 0.68 | 0.67 | 0.81 |
| Casein specific IgE level | |||||
| KU/L | 0.97 | 1.22 | 3 | 2.39 | 2.73 |
| SE | 0.68 | 0.70 | 0.79 | 0.90 | 0.85 |
| SP | 0.81 | 0.63 | 0.80 | 0.78 | 0.83 |
| PPV | 0.80 | 0.80 | 0.90 | 0.90 | 0.95 |
| NPV | 0.70 | 0.51 | 0.62 | 0.78 | 0.55 |
| Ef | 0.74 | 0.68 | 0.79 | 0.86 | 0.84 |
| Prev | 0.52 | 0.67 | 0.69 | 0.68 | 0.81 |
SE: sensitivity; SP: specificity; PPV: positive predictive value; NPV: negative predictive value; Ef: Efficiency; Prev: Prevalence.
This multicentre, prospective four-year follow-up study of children previously diagnosed with IgE-mediated allergy to CMP yielded an 82 % tolerance rate after this period of time, which is higher than values reported elsewhere3,6. Garcia-Ara6 reported a 64 % tolerance rate among patients with allergy to CMP after three years of follow-up, while Vanto et al. obtained a 59 % tolerance rate among the subjects with IgE-mediated allergy after four years3.
As can be seen in these studies, the allergy to CMP is usually a transitory situation in the majority of children with allergy to CMP who follow a corresponding exclusion diet and feeding with either hydrolysed substitutes or soy formulas. Therefore, periodic follow-up must be carried out of these children in order to detect as soon as possible that allergy to CMP has been outgrown.
Several studies have examined a number of factors that could be of prognostic value in food allergy. In the case of egg allergy, one study found the main predictors to be symptoms at initial exposure and the size of skin prick tests reactions8. In the case of CM allergy, James and Sampson found neither of these parameters to be predictive of tolerance. Instead, the initial casein and beta-lactoglobulin specific IgE values were lower, and decreased significantly in patients who became tolerant10. A later study confirmed the finding that the concentration of milk specific IgE (sIgE) was lower in the group of children who became tolerant compared to those with lasting allergy5. Recently, it has been shown that the presence of IgE targeted to certain sequential epitopes on different CMP is associated with persistent CM allergy11. However, it is not predictive of the timepoint of tolerance development.
The only firm method available for determining tolerance and non-tolerance is challenge testing, which is not without risk and moreover causes discomfort for patients and their families. It is therefore desirable to develop techniques that can provide an orientation towards patient tolerance or non-tolerance status.
Although the double-blind placebo controlled oral food challenge test is the gold standard and the most appropriate method for discriminating between tolerant and non-tolerant patients12, specific IgE quantification by means of the CAP system has proved to be a useful method in the diagnosis of allergy. Its application could eliminate unnecessary challenge tests in many children6–8.
The aim of our study was to analyse the specific IgE values capable of predicting clinical reactivity to CM at the different timepoints at which the patients were evaluated. As can be seen in Tables III and IV, specific IgE is not a static value; rather, it varies over the course of follow-up, increasing in non-tolerant children compared with tolerant children. Based on the ROC curves, we analysed the sensitivity and specificity of different specific IgE cut-off values and the clinical reactivity predictive values. For a positive predictive value of 95 %, the cut-off points of specific IgE for CM and casein were seen to increase at the first three age points considered, followed by a decrease over the subsequent timepoints. These results coincide with those of other studies6.
On applying these cut-off points (PPV > 95 %) to routine clinical practice, it must be taken into account that they help us to avoid challenge testing due to the high probability that such testing will prove positive. However, they are of little help in deciding whether to perform challenge testing in patients with values below such levels, since the negative predictive value tends to be about 50 % - i.e., approximately one-half of all challenge tests below these levels will prove positive. In routine clinical practice our greatest interest is to define a method capable of detecting tolerance as soon as possible. Due to the low NPV of each point, we have sought other cut-off points offering greater diagnostic efficiency, i.e., those where sensitivity and specificity are more balanced, with an increase in NPV even at the expense of a decrease in PPV.
Casein is one of the major allergens responsible for CMP allergy13, and seems to play an important role in persistent allergy5. In the initial diagnosis of allergy to CMP in the first year of life, in which different cut-off points of specific IgE to CMP were analysed, the predictive value of CMP showed little improvement over specific IgE to milk14. Nevertheless, it was observed in the follow-up of these children that casein is the protein that best discriminates between persistence of allergy and tolerance6.
In this study, measurement of IgE specific for casein could not be shown to yield more prognostic information than that obtained by the measurement of IgE specific for CM, except at the 48months cut-off point. The cut-off point corresponding to 36months, 2.2 kUA/l for CM, offered the greatest diagnostic efficiency values, along with 2.7 kUA/l for casein after 48months. Thus, with specific IgE values for CM of > 2.2 kUA/l, the clinical reactivity probability would reach 92 % in the third year of life. In addition, at that age, high NPV values are reached, i.e., values < 2.2 kUA/l for CM would have a probability of tolerance of up to 80 %. This cut-off point is lower than that reported in another study6 in which similar diagnostic efficiency was obtained (0.88) with levels of 14 kUA/l corresponding to specific IgE for CM. These values are not comparable, due to the different prevalences in both studies.
Sampson7, in his prospective study, calculated the predictive value corresponding to a specificity of 90 %. This value for CM would be about 15 kUA/l in patients with ages between 3months and 14years. According to this study, challenge tests in a child with specific IgE to milk > 15 kUA/l would not be indicated. In contrast, challenge testing would be indicated (always under the control of a physician) if the values are between 15 and 0.35 kUA/l. A disadvantage of these cut-off points is their low NPV (53 %), which means that almost half of all challenge tests below 15 kUA/l would prove positive. This cut-off value is greater than that found in our study, though it must be taken into account that the majority of the patients in the above study had atopic dermatitis – this being a population in which IgE levels are greater as compared to patients without atopic dermatitis. Moreover, in our study the cut-off points that showed clinical reactivity would be different depending on the moment of follow-up. These data support the relevance of the age factor when specific IgE levels are assessed.
Sheck et al.15 have shown a relationship between the degree of decrease in food specific-IgE antibody concentrations over time and the likelihood of developing tolerance. However, according to the results of these authors, a large decrease in specific IgE levels is required in order to predict the likelihood of developing tolerance. For a child with CM allergy below the age of 4years at first challenge, the probability of developing tolerance was 0.66 for a decrease of 90 %, and 0.94 for a decrease of 99 % in specific IgE levels over 12months.
In conclusion, monitorisation of specific IgE levels for milk and casein by means of the CAP System in allergic children to CMPs allows us to reach high values in the prediction of clinical reactivity in the follow-up and could eliminate the need to perform oral challenge tests in a significant number of children.