INTRODUCTION
Atopic dermatitis (AD) is a chronic, relapsing, highly pruritic, inflammatory skin disease that frequently predates the development of allergic rhinitis or asthma. AD usually presents in infancy and childhood and it may persist into or even start in adulthood1. The prevalence of AD, 10-20 % in children and 1-3 % in adults, has increased two- to three-fold over the past three decades in developed countries2. Although the incidence of atopic disorders is greater in some families, genetic factors alone cannot account for the increase in the prevalence of AD. Environmental factors, in particular those related to changes in lifestyle1,2, could also be involved in the symptomatic expression of this disorder in genetically predisposed patients.
Atopic dermatitis is characterised by the presence of clinical symptoms of both IgE antibody-mediated immediate hypersensitivity and specific T lymphocyte-mediated delayed hypersensitivity. One typical characteristic is an elevated serum total and specific IgE level, occurring in 80 % of AD patients and which is called "extrinsic AD"3. Despite multiple studies the pathogenesis of AD is still not completely understood, though T cells are known to play a critical role1,2. Most T cells located in the skin in AD are of the CD45RO + memory/effector phenotype and express the selective skin-homing receptor cutaneous-lymphocyte-associated antigen (CLA)4. These cells are known to produce higher levels of Th2-type cytokines (IL-4, IL-5, IL-13), which are thought to play a key role in the pathogenesis of AD, and lower levels of Th1-type cytokines (IFN-γ)5-9. IL-4 and IL-13 are produced primarily by activated T cells and mast cells, and the genes for both these cytokines are located in the same region on chromosome 510. Both IL-4 and IL-13 can induce switching of IgG to IgE11,12. Moreover, the activation of T lymphocytes is followed by the activation of other types of cells, keratinocytes and mainly endothelial cells, which results in the production of cytokines and chemokines, enabling recruitment of inflammatory cells from the blood to the skin13.
The aim of this study was to assess the immunological mechanisms involved in AD children with acute lesions. We compared T lymphocyte markers and the cytokine production pattern, including Th1 (IFN-γ) and Th2 (IL-13), in peripheral blood mononuclear cells (PBMC) from these AD children and from non-atopic controls.
METHODS
Patients
Twenty children from the paediatric allergy Unit of Carlos Haya Hospital were selected and classified into two groups:
Ten children diagnosed with AD according to Hanifin and Rajka14 and with acute lesions (lesion onset less than three days before study), with no respiratory problems or other skin diseases. No systemic steroids, tacrolimus or psoralen-ultraviolet A had been used for at least six months prior to the study.
Ten non-atopic children with no skin or respiratory diseases, included as controls.
The study was approved by the institutional review board, and informed consent for all the diagnostic procedures was obtained from the parents of the patients and the controls.
Total serum IgE was quantified by fluoroenzymeimmunoassay (UniCAP, Pharmacia Diagnostic AB, Uppsala, Sweden) according to the manufacturer's instructions.
Phenotypic immunofluorescence analysis
PBMC from each patient were isolated by density gradient centrifugation (Nycomed, Oslo, Norway) from 6 ml of heparinized venous blood for immunofluorescence staining, as previously described15. Briefly, 5 x 105 cells were sequentially stained with different MoAbs at 4 °C. Stained cells were fixed in 1 % paraformaldehyde in PBS. Cell subsets were assessed with CD3-PerCP, CD4-APC and CD8-APC and the homing receptor expression with CLA-FITC (Becton-Dickinson, San Jose, CA).
Cytokine production was determined in cells stimulated for 4 hours at 37 °C with phorbol 12-myristate 13-acetate (PMA) (Sigma, St. Louis, MO,) and ionomycin (Sigma) at a final concentration of 25 ng/ml and 1 μg/ml of cell suspension, respectively, in which the intracellular protein transport of cytokines was disrupted with monensin (Sigma) at 10 μg/ml of cell suspension. After subset cell surface staining for 15 min, cells were fixed for 15 min using Fix and Per cell permeabilization kit (Caltag Laboratories, Burlingame, CA) and permeabilized with the same kit and stained intracellularly with monoclonal antibodies to cytokines (IFN-γ -PE and IL-13-PE, all provided by Becton-Dickinson) for 30 min at room temperature. After washing, cells were analysed on the flow cytometer. A disadvantage of PMA activation is the downregulation of certain surface proteins, including CD4. Therefore, as most CD4+ T cells are contained within the CD3+ /CD8 population, when we refer to the CD4+ population in the analysis of the cytokine production we in fact measured the CD3+ /CD8 population. Six-parameters were analysed on a Facscalibur flow cytometer using Cell Quest software. Negative isotype controls were used to verify the staining specificity of the antibodies used.
Statistical study
Data are presented as box-plots displaying medians and interquartile ranges (IR). As the variables evaluated were not distributed normally, the mean comparisons were done by non parametric analysis (Kruskall-Wallis and, if significant, Mann-Whitney tests). All reported P values represented two-tailed tests, with values of 0.05 or less considered statistically significant. The statistical analysis was performed using the SPSS program version 11.5.
RESULTS
Comparisons between AD patients and controls showed that total IgE was significantly higher in AD patients (median: 886 IKU/L; IR: 2626-299 IKU/L) compared to controls (median: 53.3 IKU/L; IR: 103-26 IKU/L; p = 0.01).
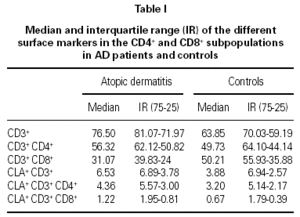
The percentages of the total CD4+ and CD8+ subpopulations in CD3+ cells and in CLA+ cells are presented in table I. We detected an increase in the percentage of CD3+ cells in AD children compared to controls (p = 0.015), and a decrease in the CD8+ subset in AD patients (p = 0.023). The CD4+/CD8+ ratio was significantly increased in AD patients compared to controls (p = 0.04). There were no differences in the CLA expression in peripheral blood T cells between both groups.
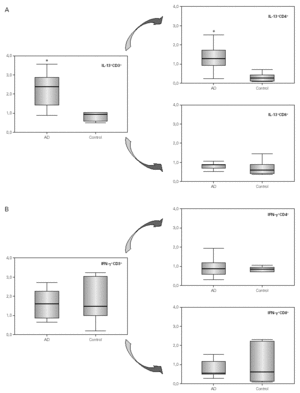
The median and IR of the percentage of CD4+ and CD8+ subpopulations expressing IL-13 and IFN-γ are shown in figure 1. Comparisons of cytokine production showed an increase in the IL-13 production in CD3+ cells (p = 0.01), and only detect in the CD3+ CD4+ cells (p = 0.001). No difference in the IFN-γ production between AD children and controls was detected. Figure 2 shows flow cytometry examples of IL-13 and IFN-γ production in the CD4+ and CD8+ subpopulations from one child in each group.
Figure 1.--Box plot of the percentage of IL-13 (A) and IFN-g (B) production in CD3+ , CD4+ and CD8+ subpopulations. Comparisons have been made between AD children and controls. Statistical differences (*) are considered significant with a p < 0.05.
Figure 2.--Flow-cytometry of the cytokine production values of IL-13 and IFN-γ in the CD4+ and CD8+ subpopulations, in one patient with AD and one control.
We evaluated the relationship between IL-13 production and total IgE levels. As IL-13 production was only shown in the CD4+ subsets we compared the production of IL-13 in CD4+ cells in all subjects (AD patients and controls) categorized by the total IgE level (fig. 3). The categories were IgE < 100 IKU/L, from 100 to 1.000 IKU/L and > 1.000 IKU/L. Results showed that IL-13 production was significantly increased in subjects with higher IgE levels, with a p = 0.03 between the first two categories and p = 0.017 between the first and the third categories. Although the difference between the second and the third category was not significant there was a tendency to increase.
Figure 3.--Box plot of the percentage of IL-13 production at different total IgE levels. Comparisons have been made between different total IgE levels. Statistical differences (*) are considered significant with a p < 0.05.
DISCUSSION
Many studies indicate that AD is the cutaneous manifestation of a systemic immunological disorder named atopy, and this term describes the genetic predisposition to IgE-mediated allergies. B cell production of IgE requires the T cell derived cytokines IL-4 and/or IL-13, whereas IFN-g inhibits this IgE synthesis16. In order to understand the mechanisms involved in AD we evaluated several lymphocyte markers and the cytokine production, including Th1 (IFN-g) and Th2 (IL-13), in PBMC from a group of AD children with acute lesions and a group of non-atopic children as controls. We chose IL-13 instead of IL-4 for defining a Th2 pattern because IL-13 is produced in rapid response to activation of peripheral blood T cells and maintained over a longer period of time whereas IL-4 secretion is more transient17.
As has been described in skin biopsies18, in our patients we found an increase in CD3 + cells and an increase in the CD4 + /CD8 + ratio. Although T cells specific for skin related allergens are known to be confined to the CLA+ T cell population19, we found no differences in the expression of T cell skin homing receptors in these AD children with acute lesions compared with the controls. These findings, which agree with previous results from our group20, may be explained by the fact that in the initial stage of the reaction the CLA lymphocytes are located in the skin, with lower levels in peripheral blood.
We used flow cytometry to analyse the frequencies of CD3+ , CD4+ and CD8+ cells expressing IL-13 and IFN-γ. The flow cytometer has the advantage of being able to study cytokine-producing cells at the single cell level. Thus, we were able to analyse which CD4 or CD8 subpopulation was producing these cytokines. In the AD children we detected a typical Th2 cytokine pattern with increased IL-13 production in T lymphocytes. Classification of these T lymphocytes into CD4+ or CD8+ showed that this increase only occurred in the CD4+ subpopulation. This agrees with other authors who have also found this increased in PBMC upon stimulation with superantigen21, with antiCD322 or in skin lesions provoked by epicutaneous application of allergens19,21. Moreover, this IL-13 expression has been mainly detected in skin biopsies obtained from acute lesions23, which is in agreement with our results. Although a decrease could be expected, we detected no significant changes in the production of IFN-γ in either the CD4+ or the CD8+ subsets. As increased level of IFN-γ had been detected in chronic AD skin lesions24 and it is thought that this cytokine may be involved in the pathogenesis of AD1,2 our results are not so surprising.
As previously reported by others11,12,25, we found that the subjects with higher total IgE levels were those who were producing higher levels of IL-13. This is in consonance with the role of IL-13 in the induction of IgE synthesis.
In conclusion, in children with acute atopic dermatitis lesions there is an increase in CD4+ cells producing the Th2 cytokine IL-13, and that there is a close relation between this cytokine production and total IgE levels. This indicates that, at least in its extrinsic form, atopic dermatitis is a cutaneous manifestation of allergy.
ACKNOWLEDGEMENTS
We thank Ian Johnstone for help with the English language version of the text.
This study was partly supported by a grant from the Spanish Ministry of Health (01/0865).
*Premio SEICAP. Congreso de Marbella, 2003.