Drug provocation tests in children are always a problematic task. In the present article the most important aspects of this technique are reviewed, including the differences between children and adults; the main mechanisms involved in drug reaction; how to perform the different tests; and when they are indicated.
“Primun non nocere”. The first consideration is not to harm. Although it is frequent to come across phrases such as: “The growing use of drugs increases the number of adverse drug reactions (ADRs),1 and therefore of iatrogenic complications”, there is a considerable lack of knowledge of such reactions – not because of a lack of studies, which have increased in number in adults in recent years and have remained stable in the paediatric population (Table 1), but because of a lack of consensus regarding the methodologies and tests used, which makes it difficult to establish comparisons.
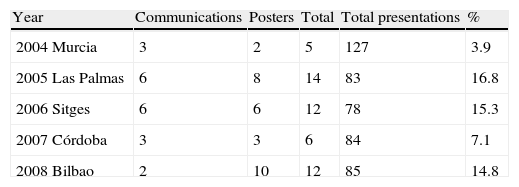
Congresses of the Spanish Society of Paediatric Allergy and Clinical Immunology (SEICAP) in the last 5 years and list of presentations relating to adverse drug reactions (ADRs)
| Year | Communications | Posters | Total | Total presentations | % |
| 2004 Murcia | 3 | 2 | 5 | 127 | 3.9 |
| 2005 Las Palmas | 6 | 8 | 14 | 83 | 16.8 |
| 2006 Sitges | 6 | 6 | 12 | 78 | 15.3 |
| 2007 Córdoba | 3 | 3 | 6 | 84 | 7.1 |
| 2008 Bilbao | 2 | 10 | 12 | 85 | 14.8 |
Drug allergies are more common in adults, in contrast to other allergic disorders such as dermatitis, food allergy, rhinitis or asthma, which are more frequent in the paediatric population.
Among adults, drug allergies are more common in females than in males. No gender differences have been identified among the children evaluated for suspected ADRs.1 These data coincide with the findings of other studies conducted in paediatric populations,2–4 although a male predominance has been observed in a study carried out by our Service.5
Children moreover suffer more infections than adults. Based on our sources, in a series of 135 children, only 18% referred no infectious process prior to administration of the causal drug.5
While the true incidence of ADRs in the general population is not precisely known, the Alergológica 2005 report6 indicates that 14.7% of all patients who report to the allergology clinic for the first time do so because of some drug reaction. Specifically, as regards antibiotics (the main drugs responsible for allergic reactions), approximately 15% of the population refers reaction to penicillin. Among these individuals, however, only 15–20% are confirmed as having true allergy to the drug. In fact, there are no ADR databases – only partial studies7 which indicate that the most frequently implicated drug substances in childhood are the following: beta-lactam antibiotics (implicated in 79.7% of all cases) (amoxicillin 38.3%; amoxicillin-clavulanate 40.2%; cephalosporins 19.8%; penicillin 1.6%); anti-inflammatory drugs (11.8%) (ibuprofen 61%; paracetamol 17.7%; metamizol 21.3%; aspirin 11.0%); other antibiotics (3.3%); other drug substances (3.2%); and topical anaesthetics (1%). In 1% of the cases the patient is unable to remember the causal drug.
Skin manifestations are the most common type of reaction, with negative allergological study findings in most cases. This indicates that the symptoms are related to the infectious process, not to hypersensitivity to the administered drug substance. Similar data have been published by other authors,8,9 with an overwhelming presence of skin manifestations7: skin symptoms are present in 96.2% of the cases (nonspecific exanthema or rash 47.9%; urticaria 38.3%; angio-oedema 17.1%), with gastrointestinal manifestations in 11.2%, and anaphylaxis in 0.4%. Low incidences are reported for serum sickness, autoimmune processes (haemolytic anaemia secondary to drugs, agranulocytosis), organ involvement such as pulmonary infiltrates with eosinophilia, or acute interstitial nephritis. In paediatric practice, and as an exception to the usual reactions, special mention should be made of serum sickness, which may be caused by cefaclor. Since 1970 there have been reports of clinical conditions similar to serum sickness following the administration of this drug. The manifestations appear at the end of the first week or in the second week of treatment, and are characterised by fever, erythema multiforme and arthropathy. These symptoms may worsen despite therapy, and the skin condition tends to resolve within 2–8 weeks.10
Definition and classificationIn 1968 the World Health Organisation (WHO) defined adverse drug reactions as the “harmful or undesired effects that appear with the doses used in humans for preventive, diagnostic or therapeutic purposes”. Such reactions have been classified in different ways, although the most widely used classification considers two major groups:
I. Type A reactions: These are a consequence of the direct or indirect pharmacological effects of the medication. As such, they are usually predictable and dose-dependent, affect a greater proportion of the population, and can be largely prevented. Type A reactions are believed to represent 70–80% of all adverse drug reactions, and include the following:
- 1.
Overdose. Alterations in the release, absorption, distribution and elimination of drug substances can increase their bioavailability, thereby contributing to increase the plasma drug levels. Such alterations can be caused by interactions with other drug substances.
- 2.
Collateral effects. All drugs have more than one action, even though only one of them may be desirable. Such collateral effects are very well known in some cases (e.g., constipation induced by codeine), while others are less well known and can be confused with allergic reactions (e.g., non-specific histamine release induced by morphic agents can induce skin reddening).
- 3.
Side effects. These are an indirect consequence of the primary action of a given drug and are not seen in all patients. An example is the appearance of candidiasis as a result of antibiotic use.
II. Type B reactions: These are unrelated to the pharmacological actions of the drug, and as such are unpredictable. Type B reactions are only seen in especially susceptible individuals, and in most cases are discovered in the post-marketing phase of the medication. They are generally infrequent, but tend to be serious. The following reactions are included in this category:
- 1.
Idiosyncratic reactions. These are qualitatively abnormal responses, different from the pharmacological action of the drug, and in which genetic mechanisms are implicated. These reactions include genetic alterations in drug metabolism (slow acetylators, limited oxidation capacity of the hepatocyte microsomal P450 enzyme system, enzyme deficiencies, etc.).
- 2.
Allergic or hypersensitivity reactions. These reactions are seen in a limited number of patients, are unrelated to the pharmacological action of the drug, and are generated via an immunological mechanism.11
Adverse drug reactions generated via an immunological mechanism occur when previous or continuous exposure to one same drug substance or to a structurally related drug substance stimulates the production of specific antibodies, sensitised T lymphocytes, or both. These reactions generally affect a small number of patients, and usually manifest in response to drug doses that are lower than those required to obtain the pharmacological effect.12
Drugs are exogenous molecules recognised by the body as foreign substances. As a result, an immune response is generated in many cases. Drug immunogenicity increases with increasing molecular size and complexity. In this context, macromolecular drugs such as proteins and peptidic hormones are strongly antigenic. However, most drug substances are haptens, and their potential for inducing an allergic response depends on their capacity to acquire antigenicity upon covalently binding to macromolecules – generally proteins.13
A number of criteria must be met in order to regard an adverse drug reaction as representing an allergic response:
- •
The clinical manifestations must be unrelated to the pharmacological effects of the drug.
- •
The reaction must be reproducible in the same patient after the administration of minimal doses of the drug or of chemically related substances.
- •
There must have been at least one previous exposure to the drug, with good tolerance. The time elapsed from such initial exposure to sensitisation may be variable (days to years), although at least 5–10 days must have past from first exposure (sensitising dose) to administration of the reaction-inducing dose (triggering dose).14
Drug-induced allergic reactions can be generated via any of the four immunological reactions described by Gell and Coombs, although the great majority are mediated by specific IgE or T cells.
- 1.
Type I reaction, immediate hypersensitivity or anaphylactic reaction. These are the most frequent allergic drug reactions, and are the result of antigen binding to its specific IgE antibody.20,26
- 2.
Type II reaction, cytotoxic or cytolytic antibody reaction. These reactions are mediated by interaction between the antigenic determinants of the drug molecule present on the surface of different cells and preformed circulating antibodies (IgG and IgM, and to a lesser extent IgA).15
- 3.
Type III reaction, mediated by immune complexes. These reactions occur when antigens from circulating drug molecules react within the tissues with soluble antibodies (fundamentally IgM), giving rise to immune complexes that form microprecipitates upon the endothelium of small blood vessels – causing secondary cellular damage.16
- 4.
Type IV reaction, delayed hypersensitivity reaction. These are reactions that occur once 24h have elapsed since administration of the drug. T lymphocytes, NK cells and monocyte-macrophage cell lines participate in these reactions.17
Reactions involving a possible and unclear immune mechanism. A number of reactions remain in which intervention of the immune system (particularly T cells) has always been suspected. These include delayed exanthema or rash, fixed exanthema, erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis, exfoliative dermatitis (erythroderma), pulmonary infiltrates with eosinophilia, nephritis and vasculitis. At present, these conditions are referred to as reactive or active metabolite syndromes, or idiosyncratic drug reactions. Such reactions do not necessarily require prior exposure to the drug, although when they manifest upon first exposure to the drug substance they always do so a certain time after administration of the latter.18
From a practical perspective, use is made of the chronological classification proposed by Levine,19 which relates the condition to the time elapsed from administration of the drug to appearance of the reaction:
- •
Immediate reactions (appearing in under 1h).
- •
Accelerated reactions (appearing within 1–72h).
- •
Late reactions (appearing more than 72h after exposure to the drug).
Correct clinical history compilation. “More objective data, improved diagnosis”.
A common problem in paediatrics is to determine whether a skin rash is an allergic drug reaction or the clinical manifestation of a viral infection. This is particularly the case in children less than 5 years of age, where most fever processes are of viral nature involving different skin manifestations. Such patients with febrile processes are sometimes treated with antibiotics despite the lack of evidence of a bacterial origin. Skin rash commonly manifests in the course of the illness; as a result, allergy is suspected and the medication is suspended even when it is the treatment of choice. Alternative and more expensive drugs in turn are often prescribed that may be less indicated and produce more side effects. Correct evaluation of the child is therefore essential, based on a thorough allergological study, with a view to avoiding wrong diagnoses.20,21 Allergic reactions have a number of characteristics, including: (a) no relation to the pharmacological effect of the drug; (b) the existence of a variable symptoms-free interval before onset of the clinical manifestations; (c) the appearance of clinical manifestations acknowledged as being of an allergic nature; and (d) resolution of the symptoms upon suspending the drug. On the other hand, it is accepted that repeat administration of the drug, or of another drug of similar structure, induces reappearance of the symptoms. As a result, even with correct compilation of the patient case history, a controlled provocation (exposure) test usually constitutes a key diagnostic tool.
An aspect requiring consideration is the evaluation of the mechanisms involved in immunological adverse drug reactions (ADRs) in children (IgE mediated or otherwise), and to search for immunological patterns6 allowing us to establish a clear differential diagnosis between viral skin rash and drug-induced skin symptoms, since both interact with the immune system.22
Clinical historyA guided, consensus-based case history is to be compiled.23 Ideally, the protocol used should be the same for all centres that study ADRs. However, even a thorough case history offers limited sensitivity, despite the inclusion of detailed information on the suspect drug or drugs, the disease for which treatment was indicated, the time since administration of the drug, description of the symptoms and their duration, the need for treatment to control the manifestations (antihistamines, corticosteroids, etc.), and the report of the emergency ward in which the patient was attended (particularly in the event of life-threatening conditions).
The case history is often able to yield sufficient information to establish the diagnosis, and its sensitivity and specificity can be increased when the history is compiled during the acute phase and tryptase assay is possible.24 In this context, tryptase is a very useful marker for evaluating specific IgE hypersensitivity drug reactions, and can even be assayed in serum after death as a fatal anaphylaxis marker. Evaluations can also be made of histamine release and eosinophilia, though there are no ADR-specific clinical symptoms or laboratory test findings.25
Only 5–10% of all patients who claim to have ADRs actually have such reactions.26 On referring these data to the paediatric population, the case history was found to be suggestive of an allergic reaction in 19.5% of the cases, questionable in 16%, and not suggestive of an allergic reaction in 64.5%. The diagnosis of allergy was confirmed in 3.7% of the cases.27 In contrast, children not seen in a Service of Allergy were classified as presenting drug allergy without adequate justification – thus assuming a wrong diagnosis for years.
DiagnosisSince the 1960s, the case history has been the principal diagnostic tool in IgE-mediated reactions,28 along with controlled provocation testing. There are three basic types of provocation tests: intraepidermal (prick test), offering moderate sensitivity; intradermal (ID), offering improved sensitivity but poorer specificity; and epicutaneous (patch test), which is difficult to apply. In all three techniques immediate and delayed readings can be evaluated.
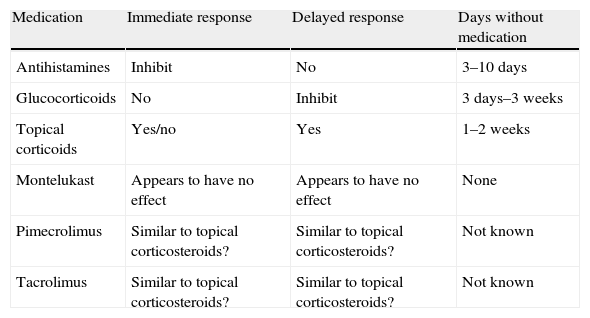
RecommendationsDuring skin testing, the child must be free of fever and inflammatory processes which alter skin reactivity, and drugs such as those reflected in the table are to be avoided (Table 2).
Recommendations during skin testing
| Medication | Immediate response | Delayed response | Days without medication |
| Antihistamines | Inhibit | No | 3–10 days |
| Glucocorticoids | No | Inhibit | 3 days–3 weeks |
| Topical corticoids | Yes/no | Yes | 1–2 weeks |
| Montelukast | Appears to have no effect | Appears to have no effect | None |
| Pimecrolimus | Similar to topical corticosteroids? | Similar to topical corticosteroids? | Not known |
| Tacrolimus | Similar to topical corticosteroids? | Similar to topical corticosteroids? | Not known |
If skin testing is performed with high molecular weight drugs (e.g., insulin) reliability is greater than when a hapten is used. In most cases, however, no commercial preparation is available, and testing must be made by dilution to the irritative cut-off point.
On the other hand, it must be taken into account that while the elicited reactions are regarded as harmless, there have been reports of systemic reactions – particularly with intradermal testing (anaphylaxis).29,30
As to whether dilutions can be prepared for several days, data are lacking, though application appears to be valid for a period of one week.31 Such dilutions are made for any type of drug, although the parameters have only been well established for the penicillins.32 Repeat testing within a month is advised if the patient history proves suspicious. Most groups that work with ADRs use time intervals between 3 weeks (minimum) and three months (maximum) after the time of the reaction.33
The test is to yield an immediate reading34 after 20–30min, and a late reading35 after 48–72h, according to the described techniques.36 In up to 17% of all cases the readings are difficult to interpret.37 In general, a positive immediate reading is indicative of an IgE-mediated reaction, although the potential for false-positive results must be taken into account (attributable to the use of a non-physiological solution causing skin irritation or to a histamine-releasing drug substance). In turn, a negative test does not rule out any possibility, though the following situations must be considered: (a) selective IgE response or untested metabolite; (b) loss of test sensitivity due to the time elapsed since the reaction took place38,63; and (c) loss of skin sensitivity due to the administration of concomitant medication.
Skin testing in turn is not useful in application to the following disorders: drug-induced lupus erythematosus, drug-induced kidney or liver disorders, vulgar pemphigus, and interstitial lung disease.
The prick test must be negative before intradermal testing is carried out.39
In the case of non-immediate reactions, attempts are made to determine whether epicutaneous (patch) testing should accompany, replace or complement intradermal testing. With the exception of drug-induced fixed exanthema and sulphamide studies, where testing has been consolidated, experience in paediatric patients is lacking.40
Controlled exposure testsThese tests have also received other names, such as drug provocation testing or tolerance testing. The term “controlled exposure” is gaining increased acceptance, since the other two terms (provocation and tolerance testing) precondition the result of the test.
DefinitionControlled exposure testing is the “controlled administration of a drug to confirm or discard allergy”, and constitutes the gold standard for evaluating ADRs. This is because skin testing and in vitro tests such as the radioallergosorbent test (RAST) can help diagnose immediate hypersensitivity reactions mediated by IgE antibodies, and particularly reactions to beta-lactam antibiotics.41 In other types of reactions mediated by T cells, skin testing offers only low sensitivity, and the in vitro tests of cell proliferation in response to drugs that are currently available do not offer important specificity in terms of the response obtained.42
Indications- 1.
To discard drug hypersensitivity in patients with non-suggestive or inconclusive case histories.
- 2.
To offer a safe alternative in the case of hypersensitivity.
- 3.
In the case of several drugs, each of them must be tested, beginning with the substance least likely to induce a reaction.
- 4.
To assess related drug cross-reactivity.
- 5.
To establish a firm diagnosis.
While little consensus has been reached, the most widely used protocol is that developed by the European Academy of Allergology.43 A first consideration is the patient informed consent form – this being an essential legal instrument for starting the study.
Informed Consent. Model proposed by the ADR Committee for paediatric patients.
(Figure 1 In compliance with underage patient rights, and in abidance with Spanish General Health Law (25/4/1986) Art. 10.)
Methodology of controlled exposure testing- 1.
Risk/benefit assessment. Consideration is required of the need for a given medication and its alternatives for a given disease process, together with the benefits of continuing to use the same medication and the potential risk of worsening of the background disease – particularly in children with chronic illnesses.
- 2.
Protocol to be applied. The child must be in good health and if possible should be taking no medication (evaluate medication for chronic disease). While not firmly established, the following principles are accepted: fast-acting, and delayed action antihistamines are to be avoided in the previous three and 14 days, respectively. As regards oral corticosteroids, where the guidelines are least firmly established, suspension should be carried out three days before testing, except when corticosteroid dosing has lasted for over three weeks – in which case suspension should be carried out at least one week before. Montelukast should be suspended three days in advance only if the study is related to non-steroidal anti-inflammatory drugs (NSAIDs) and asthma.44 The rest of the drugs commonly used in paediatric patients, such as antibiotics, anti-fever medication, mucolytic formulations, antitussive drugs, etc. should be suspended three days before testing, due to the possibility of interferences.
- 3.
Where testing should be made. An in-hospital setting is indicated in all cases, with the usual guarantees for control and management of the possible complications.
- 4.
Who should perform testing. Trained personnel should perform the tests, i.e., physicians and nurses.
- 5.
Required material and medication. No protocol has been established, although there are some data45 generated by the ENDA group and also other publications on the subject.46 Oxygen, adrenalin, bronchodilators, steroids, antihistamines, intravenous saline and resuscitation material are needed, as well as means for aspiration, catheterisation, pulsioxymetry and blood pressure determination.
- 6.
Anaphylaxis management protocol
- 7.
Preparations prior to testing. One week before testing, the Pharmacy Service should be asked to supply the required drugs and placebo, and the patient and medication identifying labels (including preparations and dilutions). Signing of the informed consent by the patients or their legal caretakers should be checked, with confirmation that they do not wish to cancel the test or part of the test (e.g., performing only skin tests). It must also be confirmed that the patients have not again taken the medication targeted for testing, and that they suffer no acute disease processes.
- 8.
Administration route. The same route as that which caused the reaction is to be used. The tendency in children is to use the oral route, though it may delay drug absorption. The degree of absorption when using the intramuscular route is not known, and this route is almost exclusively reserved for vaccine calendar exposure/tolerance tests. The subcutaneous route is little used (e.g., in application to local anaesthetics). Lastly, the intravenous route is the option of choice in application to antibiotics that are not administered orally (e.g., ceftazidime, vancomycin).
- 9.
Commercial drugs without mixtures should be used – preferably the same medication that caused the reaction, since in some instances the additives may vary (e.g., 2% and 4% ibuprofen).
- 10.
Medical form: Type of test, type of reaction, name of the drug, administered dose and millilitres of each dose, time intervals, the need or not to continue taking the medication at home, and the number of days of treatment. Nursing form: Name of the patient, type of test, personal data, number of doses to be administered, timing of administration, contact telephone number, and the provision of clear written home instructions.
- 11.
Final report: Once the study has been completed, a written medical report is to be prepared,46 regardless of whether the results have been positive or negative. The report must be clear and concise, and should include treatment alternatives. If the patient proves allergic, emergency medication is to be indicated (in our case we recommend self-injectable adrenalin, among other agents).
Ambiguous recommendations such as “Although the test results are negative, caution is required with…” are to be avoided. If the patient fails to complete the study, a report should still be produced, informing of the allergy results of the implicated medications and recommending the avoidance of such drugs.
The following is normally used:
First dose 1/100
Second dose 1/10
Third dose 1/1
Note: In practical terms, the first and second doses are not subtracted from the third; therapeutic doses should always be used.
Interval between doses: 1h.
Timing: Dosing should start early in the morning (our experience indicates that the process lasts longer than theoretically expected).
Waiting time: Two hours after the last dose, except in the case of non-steroidal anti-inflammatory drugs, where the time is extended to three hours. Where dictated by the situation, the Emergency Service can be informed. When testing continues in the home, the patient should take the medication for the same number of days as when the reaction took place,47 and adequate medication should be provided in the event of a reaction: dexchlorpheniramine 0.15–0.20mg/kg/day (0.5ml/kg/day and oral corticosteroids in the form of prednisolone 0.15–2mg/kg/day.
Re-provocation- •
In the case of a suggestive history and negative study results.
- •
In the presence of a prolonged period of time between the study and provocation testing (over 2 years).
- •
Before starting, a RAST should be requested (if available) 6 weeks after the study; if not available, intradermoreaction should be repeated. If the implicated drug is of obligate prescription, as in the case of the calendar-based vaccines, testing of tolerance or desensitisation is indicated.48
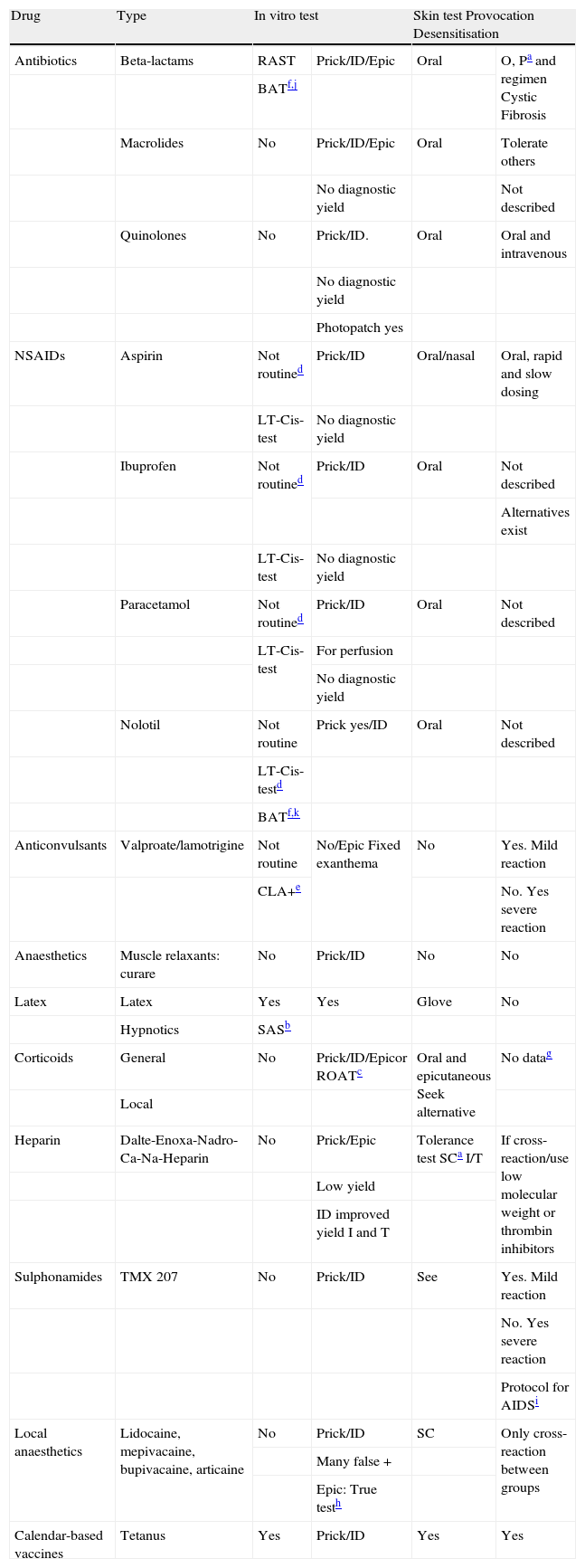
Table 3 below summarises adverse drug reaction testing in relation to the drugs most commonly used in paediatric practice.
Adverse drug reaction testing in relation to the drugs most commonly used in paediatric practice
| Drug | Type | In vitro test | Skin test Provocation Desensitisation | ||
| Antibiotics | Beta-lactams | RAST | Prick/ID/Epic | Oral | O, Pa and regimen Cystic Fibrosis |
| BATf,j | |||||
| Macrolides | No | Prick/ID/Epic | Oral | Tolerate others | |
| No diagnostic yield | Not described | ||||
| Quinolones | No | Prick/ID. | Oral | Oral and intravenous | |
| No diagnostic yield | |||||
| Photopatch yes | |||||
| NSAIDs | Aspirin | Not routined | Prick/ID | Oral/nasal | Oral, rapid and slow dosing |
| LT-Cis-test | No diagnostic yield | ||||
| Ibuprofen | Not routined | Prick/ID | Oral | Not described | |
| Alternatives exist | |||||
| LT-Cis-test | No diagnostic yield | ||||
| Paracetamol | Not routined | Prick/ID | Oral | Not described | |
| LT-Cis-test | For perfusion | ||||
| No diagnostic yield | |||||
| Nolotil | Not routine | Prick yes/ID | Oral | Not described | |
| LT-Cis-testd | |||||
| BATf,k | |||||
| Anticonvulsants | Valproate/lamotrigine | Not routine | No/Epic Fixed exanthema | No | Yes. Mild reaction |
| CLA+e | No. Yes severe reaction | ||||
| Anaesthetics | Muscle relaxants: curare | No | Prick/ID | No | No |
| Latex | Latex | Yes | Yes | Glove | No |
| Hypnotics | SASb | ||||
| Corticoids | General | No | Prick/ID/Epicor ROATc | Oral and epicutaneous Seek alternative | No datag |
| Local | |||||
| Heparin | Dalte-Enoxa-Nadro-Ca-Na-Heparin | No | Prick/Epic | Tolerance test SCa I/T | If cross-reaction/use low molecular weight or thrombin inhibitors |
| Low yield | |||||
| ID improved yield I and T | |||||
| Sulphonamides | TMX 207 | No | Prick/ID | See | Yes. Mild reaction |
| No. Yes severe reaction | |||||
| Protocol for AIDSi | |||||
| Local anaesthetics | Lidocaine, mepivacaine, bupivacaine, articaine | No | Prick/ID | SC | Only cross-reaction between groups |
| Many false + | |||||
| Epic: True testh | |||||
| Calendar-based vaccines | Tetanus | Yes | Prick/ID | Yes | Yes |
ROAT: Repeated open application test. If epicutaneous testing proves negative, purportedly implicated commercial corticosteroid is used twice a day for 7 days at the reaction site or anterior surface of the forearm.
LT-Cis-test: Cysteinic leukotriene test. From: Weck AL. Cellular allergen simulation test (CAST); a new dimension in allergy diagnostics. ACI News 1993; 1(5); 914.
CLA: Cutaneous lymphocyte-associated antigen. From: Leiva L, Torres MJ, Posadas S, Blanca M, Besso G, Valle F et al. Anticonvulsant-induced toxic epidermal necrolysis. Monitoring the immunologic response. J Allergy Clin Immunol 2000;105(1/1);157-165.
Desensitization described with hydrocortisone. From: Clee M, Ferguson J, Browning M, Jung R, Clark R. Glucocorticoid hypersensitivity in asthmatic patient: presentation and treatment. Thorax 1985,40:477-478.
True test. Commercial mixture, includes the standard series of the GEIDEC. Contains 5% lidocaine, 1% procaine chloride, 5% cinchocaine chloride, 1% amethocaine chloride, and 1% benzocaine in vaseline.
From: Yoshizawa S, Yasuoka A, Kikuchi Y. A 5-day course of oral desensitization to TMX is successful in patients with human immunodeficiency virus type 1 infection who were previously intolerant but had no TMX specific IgE. Ann Allergy Asthma Immunol 2000;85;241-244.
Lack of adequate guarantees for correct testing.
Drugs in disuse or of doubtful efficacy.
Patient-dependentRefusal to sign the informed consent document.
Potential risk outweighing the pathology caused by the medication, or previous anaphylaxis in relation to the study drug.
Special situations such as non-stabilised diabetes (can be performed with glucose excipient).
Contraindications for adrenalin use: hypertension, arrhythmias, hyperthyroidism.
Psychological alterations in the child or caretakers that may influence conduction of the test.
Serious and difficult to control background illness.
The advisability of testing should be considered when too long an interval has passed since the time of the reaction.
Testing should be postponed and posteriorly evaluated in the following casesMedication used by the patient and which may mask the results.
Acute pathology precluding correct evaluation (fever, vomiting, etc.).
Risk of gastrointestinal bleeding due to gastric erosive drug use: infrequent NSAID-induced bleeding in childhood.
Mallory-Weiss syndrome.
Chronic urticaria.
Uncontrolled asthma.
Testing should not be performed in the following cases:Relevant toxicoderma. Such skin conditions include the following:
- 1.
Drug-induced fixed exanthema: Single or multiple red-violet lesions on skin and/or mucosal membranes (typically in genital region), always appearing in the same location on administering the causal medication. These lesions are produced particularly by NSAIDs and sulphamides.
- 2.
Steven-Johnson syndrome: Generalised skin rash with characteristic bull's-eye lesions that also affect the mucosal membranes. NSAIDs, sulphamides, penicillins and anticonvulsants are the most commonly implicated drugs.
- 3.
Toxic epidermal necrolysis or Lyell syndrome: This is the most serious skin reaction produced by drugs, although its frequency is very low. The patients have the appearance of major burn victims. The mortality rate is high (in the range of 30%). The condition is particularly associated with NSAIDs, sulphamides, hydantoins, barbiturates and penicillins (65).
- 4.
Generalised acute exanthemic pustulosis: This condition is rare, and is characterised by the appearance of pustular lesions. The manifestations are generally mild. Many drug substances have been implicated, such as NSAIDs, cephalosporins, sulphamides, etc.
- 5.
Photosensitivity reactions: These are skin lesions that appear as a result of medication via the topical or oral route, in the context of solar exposure. Such reactions may be phototoxic (more common and with a sunburn-like appearance) or photoallergic (less common).
- 6.
Syndromes: Drug-induced rash, eosinophilia, systemic manifestations and multiorgan delayed hypersensitivity.49
IgG antibodies against beta-lactam antibiotics are produced in the early phase of the immune response to penicillins. They lack diagnostic utility in patients with beta-lactam allergy, since the general population has high titres of such antibodies. In effect, the presence of such antibodies is an indicator of beta-lactam use rather than of reaction to such drugs.50
Study of inflammatory mediatorsInflammatory mediator release is an immediate allergic response signal.51 There are a number of mediators:
- •
Histamine: This mediator is released by mast cells and basophils, and a few minutes after the reaction, peak levels are found in peripheral blood. The released histamine in turn is quickly metabolised to N-methyl histamine, which is eliminated in urine. The determination of this metabolite in urine offers a broader margin, though the technique does involve some difficulties. In effect, certain drugs such as the quinolones can interfere with the result, since they have a similar chemical structure – thus giving rise to false-positive readings. As a result, this test is not considered to be useful.
- •
Histamine release test: This test assesses in vitro histamine release by basophils obtained from peripheral blood after interaction of the haptens with the IgE antibodies bound to the cell membrane receptors. However, the diagnostic capacity of this test is insufficient.
- •
Tryptase: This is a mediator exclusive of mast cells-hence its great usefulness as an immediate allergic reaction activation marker.52 The technique offers only moderate sensitivity but great specificity. Accordingly, normal individuals have undetectable levels (<1ng/ml) of tryptase in serum or plasma, while anaphylactic patients show elevations over 5ng/ml.53
A series of cells are implicated in drug hypersensitivity reactions, although their study is presently only possible in the investigational setting.
Flow cytometry study of cell membrane markersDuring allergic reactions, lymphocytes are seen to express membrane markers including activation markers (CD25 or CD69) or homing or subpopulation markers (CD4 or CD8). Flow cytometric techniques can be used to detect the number of cells that express each of these markers, with a view to monitoring the development and course of the reactions when they are underway.
Participation of T lymphocytesIt has been seen that non-immediate drug reactions with skin involvement are characterised by an increase in the expression of cutaneous lymphocyte-associated antigen (CLA), thus confirming T cell participation in reactions of this type.54
Evaluations have also been made of the role played by lymphocytes in more serious reactions such as Lyell syndrome induced by anticonvulsants. In this context, an increase has been documented in CLA-positive T cells in parallel to the course of the disease, in both the CD4+ and CD8+ subpopulations, together with cell activation.55
Lymphocyte transformation test (LTT)This technique had fallen into disuse, due to its scant specificity. However, it has found new applicability following a series of modifications that appear to have improved its specificity and sensitivity.56–58 Basically, the test measures T lymphocyte proliferation in the presence of the antigen or hapten that induced the reaction. Such cell proliferation is expressed as a stimulation index that is regarded as positive when over a value of 3.
The test poses two fundamental problems. On the one hand, its technical difficulty requires the availability of a laboratory capable of keeping cell cultures, and on the other hand the controls are also able to proliferate due to the lymphocyte immune memory of individuals that consume medicines. Consequently, this technique is not used on a routine basis.
Marker activation studies. Measurement of cytokinesFlow cytometry can be used to measure intracellular cytokine production. Alternatively, molecular biological techniques are able to detect expression of the specific messenger RNA of each marker. In this way we are able to identify the type of reaction that has occurred, since type I reactions (involving Th2 lymphocyte activity) fundamentally induce IL-4, IL-5, IL-6 and IL-10, while delayed reactions mediated by Th1 lymphocytes produce IL-2, TNF-α and IFN-γ A recent study has reported that immediate drug reactions basically exhibit a Th2-type pattern, while late reactions are associated with a Th1-type pattern.59
Biopsies
Since most allergic drug reactions affect the skin, which is easily accessible, a skin biopsy is very useful and holds important promise for the future.60 It is the only technique allowing us to analyse the inflammatory process in situ.
Basophil activation testing applied to the diagnosis of adverse drug reactionsBasophil activation testing (BAT) has been applied to beta-lactams and metamizol. The sensitivity of BAT in application to beta-lactam allergy was found to be 52.8%, with a specificity of 92.6%. In application to metamizol, the sensitivity was 42.3%, with a specificity of 100%.
The combined use of BAT and ImmunoCAP (specific IgE) makes it possible to diagnose 65% of all patients with allergy to beta-lactams.61,62 The combined use of skin tests and BAT in application to metamizol allergy is a useful detection option in 70% of the cases. BAT has a promising future as a non-invasive in vitro diagnostic technique in patients with allergy to beta-lactams and metamizol, as well as to other drug substances.
Conflict of interestThe authors have no conflict of interest to declare.