Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome is a rare disease which can cause severe morbidity and mortality. The aim of this study is to evaluate the clinical manifestation and course of DRESS syndrome.
MethodsWe conducted a retrospective analysis of prospectively collected data in 45 patients with DRESS syndrome diagnosed between September 2009 and August 2011.
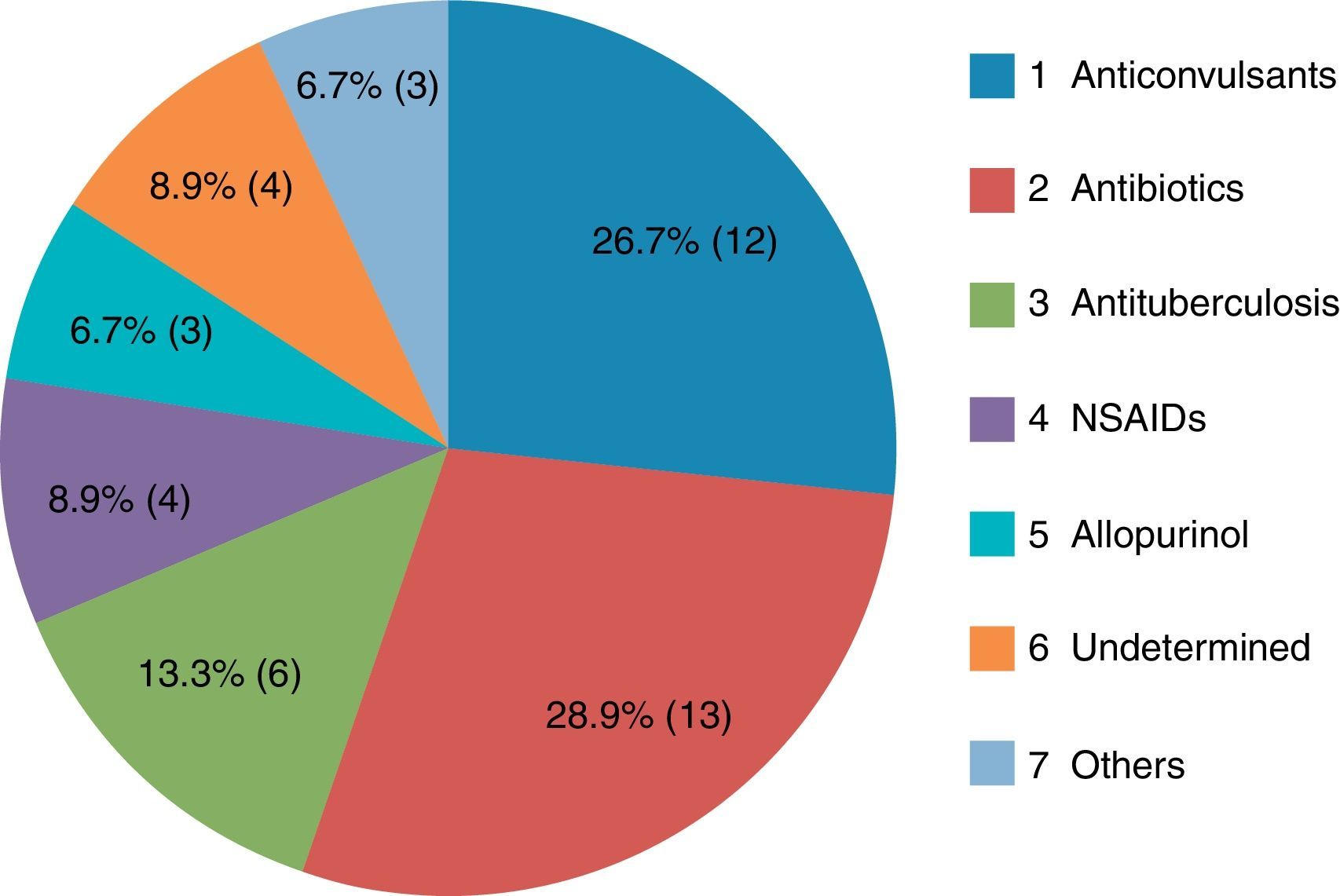
ResultsThe most common causative drug group was antibiotics (n=13, 28.9%), followed by anticonvulsants (n=12, 26.7%), antituberculosis drugs (n=6, 13.3%), non-steroidal anti-inflammatory drugs (n=4, 8.9%), undetermined agents (n=4, 8.9%), allopurinol (n=3, 6.7%), and others (n=3, 6.7%). The latency period ranged from 2 to 120 days, with a mean of 20.2±24.3 days. The longest latency period was noted for the antituberculosis drug group, at 46.5±29.9 days. Eosinophilia in peripheral blood examination was noted in 35 subjects (77.8%). Atypical lymphocytosis was noted in 16 patients (35.6%), and thrombocytopenia in seven patients (15.6%). Hepatic involvement was noted in 39 (86.7%) study patients, kidney in eight (17.8%), lung in four (8.9%), and central nervous system in one (2.3%). Systemic corticosteroids were administered to 10 patients (22.2%). Forty-three patients (95.6%) showed complete recovery, while two patients had poor outcomes.
ConclusionsDRESS syndrome was not more uncommon than generally recognised. Antibiotics were the most frequently implicated drug group, followed by anticonvulsants. Most patients with this disease showed a better clinical outcome than that which had been generally expected.
Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome is a serious drug-induced adverse reaction with a relatively long latency period. The major clinical manifestations are fever, skin rash, and the involvement of several internal organs. Various names have been used to describe this single disease1–5; this reflects the difficulties in defining the disorder and in clarifying its diagnostic criteria. Recently, the European Registry of Severe Cutaneous Adverse Reactions (RegiSCAR), a study group dedicated to investigating such reactions, has introduced the term hypersensitivity syndrome (HSS)/DRESS.6 We consider HSS/DRESS to be more compatible, because this term includes elements of the pathogenesis, aetiology and clinical features.
Originally, HSS/DRESS was first described in subjects who had been prescribed the anticonvulsant, phenytoin.7 Other drugs which have been well known as causes include carbamazepine, allopurinol, sulfasalazine, phenobarbital, lamotrigine, and nevirapine.5 Antibiotics,6 non-steroidal anti-inflammatory drugs (NSAIDs),6 and antituberculosis drugs8 have been reported sporadically as causative drugs in the form of case reports. In addition, several new drugs have increasingly been reported as new causative agents for HSS/DRESS development.
The other noteworthy feature of HSS/DRESS is a delayed onset usually 2–6 weeks after the initiation of drug therapy.3 This finding can be compatible to many traditional causative drugs, which includes anticonvulsants and antituberculosis drugs.8,9 However, after careful review of case reports including those of antibiotics, there have been some causative drugs which have had a relatively short latency period of less than two weeks.9–14
Early withdrawal of the offending drug and administration of systemic corticosteroids (SCS) has been regarded as the most important step in the treatment of this disease.15 More than 50% of study subjects were treated with SCS.16–19 However, randomised controlled trials are lacking, and whether SCS should be administered remains controversial.15
We report clinical features of 45 subjects with HSS/DRESS in a single tertiary referral centre for two years, in which we describe the characteristics of causative agents, including latency period and clinical outcomes.
Materials and methodsSubjectsWe conducted a retrospective analysis of prospectively collected data of 45 patients with HSS/DRESS syndrome, who were diagnosed between September 2009 and August 2011 in a tertiary referral centre. The clinical characteristics and courses of the patients were evaluated. This study was approved by the institutional review board of Dong-A University Hospital.
Diagnostic criteriaPatients with HSS/DRESS were defined as described previously,9 which was modified from other reports.2,20,21 This included: acute skin rash; fever of more than 38°C in a patient after taking a specific drug; at least one of the following internal organ abnormalities: lymphadenopathy at a minimum of two sites, hepatitis, nephritis, and/or pneumonitis, haematological abnormalities with eosinophilia (defined as more than 10% of total white blood cells or over 500cells/¿L), the presence of atypical lymphocytes, thrombocytopenia and/or leucopoenia. The involvement of the liver and kidney was defined by a twofold increase of liver enzymes over the normal value, and the new onset of an abnormal urinalysis with haematuria and/or proteinuria, an increasing serum creatinine above the normal range and a deterioration of pre-existing chronic renal insufficiency. Pulmonary involvement was considered when abnormal findings on chest radiographs were presented with either an increased sputum eosinophil count, improvement with SCS, or spontaneously. Central nervous system (CNS) involvement was considered when abnormal mental status was combined with radiological abnormalities in magnetic resonance imaging (MRI) and improvement after treatment with SCS. Additionally, we incorporated the RegiSCAR's6 scoring system to grade HSS/DRESS cases as “no”, “possible”, “probable”, or “definite”.
Cases of typical manifestations of Stevens–Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) were excluded. Culprit drugs were determined by reviewing medication histories and clinical courses which showed improvements of skin rash and other systemic symptoms after discontinuation of the suspected drug.22
Laboratory studiesComplete blood cell counts, serum biochemistry including liver and renal function tests, and urinalyses were performed. Serological tests for autoimmune antibodies such as antinuclear antibodies and anti-DNA antibodies, and several viral antibodies, including herpes simplex virus, cytomegalovirus, Epstein–Barr virus, HIV, and hepatitis A, B, and C viruses, were performed in order to rule out other autoimmune or viral diseases.
Statistical analysesQuantitative and qualitative results are given by the means±SD and absolute numbers or frequencies, respectively. Descriptive statistics were performed using SPSS software (ver. 15.0; SPSS Inc., Chicago, IL, USA). We used laboratory values that were the highest values during the clinical course. Comparisons of various laboratory results were performed using the Mann–Whitney U and Kruskal–Wallis tests. Correlations between various clinical parameters were evaluated with the Pearson's correlation coefficient. P values<0.05 were considered to indicate statistical significance.
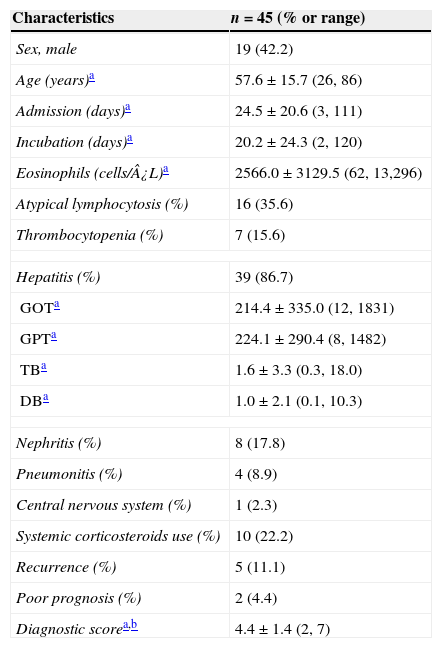
ResultsClinical characteristics of study subjectsA total of 45 patients (19 males) who were diagnosed as having HSS/DRESS were included in this study. The mean age of the patients was 57.6±15.7 years (range, 26–86) (Table 1). Among 45 study patients 26 (57.8%) were referred from adjacent primary or secondary care hospitals. Twenty-eight patients (62.2%) were treated in the department of internal medicine and others were consulted from various surgical departments. The mean point according to RegiSCAR criteria was 4.4±1.36 points (range, 2–7) (Table 1). Subjects were classified as definite 13 (28.9%), probable 19 (42.2%), and possible 13 (28.9%) HSS/DRESS cases, respectively, and none of the subjects were compatible with “no”.
Clinical characteristics of the study patients.
| Characteristics | n=45 (% or range) |
|---|---|
| Sex, male | 19 (42.2) |
| Age (years)a | 57.6±15.7 (26, 86) |
| Admission (days)a | 24.5±20.6 (3, 111) |
| Incubation (days)a | 20.2±24.3 (2, 120) |
| Eosinophils (cells/¿L)a | 2566.0±3129.5 (62, 13,296) |
| Atypical lymphocytosis (%) | 16 (35.6) |
| Thrombocytopenia (%) | 7 (15.6) |
| Hepatitis (%) | 39 (86.7) |
| GOTa | 214.4±335.0 (12, 1831) |
| GPTa | 224.1±290.4 (8, 1482) |
| TBa | 1.6±3.3 (0.3, 18.0) |
| DBa | 1.0±2.1 (0.1, 10.3) |
| Nephritis (%) | 8 (17.8) |
| Pneumonitis (%) | 4 (8.9) |
| Central nervous system (%) | 1 (2.3) |
| Systemic corticosteroids use (%) | 10 (22.2) |
| Recurrence (%) | 5 (11.1) |
| Poor prognosis (%) | 2 (4.4) |
| Diagnostic scorea,b | 4.4±1.4 (2, 7) |
GOT. aspartate aminotransferase; GPT, alanine aminotransferase; TB, total bilirubin; DB, direct bilirubin.
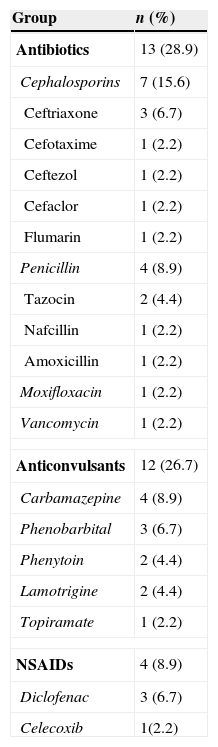
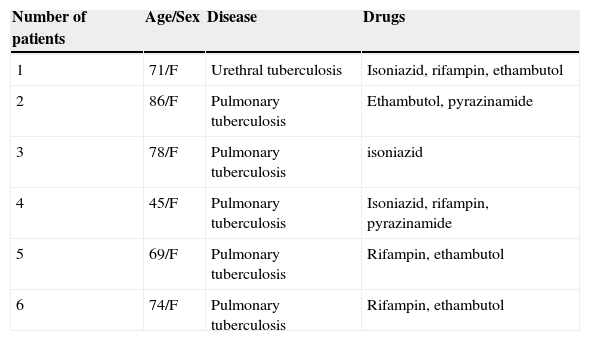
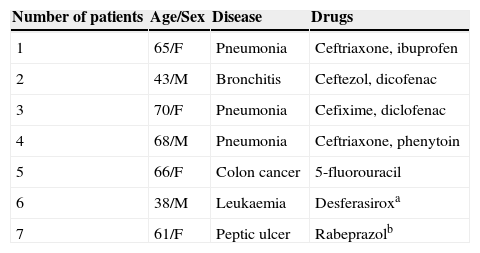
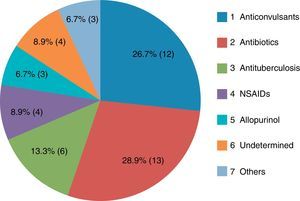
The most common causative drug group was antibiotics (n=13, 28.9%), followed by anticonvulsants (n=12, 26.7%), antituberculosis drugs (n=6, 13.3%), NSAIDs (n=4, 8.9%), undetermined (n=4, 8.9%), allopurinol (n=3, 6.7%) and others (n=3, 6.7%) (Fig. 1). Causative antibiotics included cephalosporins, penicillins, moxifloxacin and vancomycin. Anticonvulsants included carbamazepine, phenobarbital, phenytoin, lamotrigine and topiramate. NSAIDs were diclofenac and celecoxib (Table 2). Antituberculosis drugs were isoniazid, rifampin, ethambutol, pyrazinamide (Table 3). Four cases were classified as undetermined due to the concomitant use of multiple drugs, while the remaining three cases were classified as “others” due to the use of anti-cancer, iron-chelating and proton-pump inhibitors (Table 4).
Causative drugs of antibiotics, anticonvulsant and NSAIDs groups.
| Group | n (%) |
|---|---|
| Antibiotics | 13 (28.9) |
| Cephalosporins | 7 (15.6) |
| Ceftriaxone | 3 (6.7) |
| Cefotaxime | 1 (2.2) |
| Ceftezol | 1 (2.2) |
| Cefaclor | 1 (2.2) |
| Flumarin | 1 (2.2) |
| Penicillin | 4 (8.9) |
| Tazocin | 2 (4.4) |
| Nafcillin | 1 (2.2) |
| Amoxicillin | 1 (2.2) |
| Moxifloxacin | 1 (2.2) |
| Vancomycin | 1 (2.2) |
| Anticonvulsants | 12 (26.7) |
| Carbamazepine | 4 (8.9) |
| Phenobarbital | 3 (6.7) |
| Phenytoin | 2 (4.4) |
| Lamotrigine | 2 (4.4) |
| Topiramate | 1 (2.2) |
| NSAIDs | 4 (8.9) |
| Diclofenac | 3 (6.7) |
| Celecoxib | 1(2.2) |
NSAIDs; non-steroidal anti-inflammatory drugs.
Clinical characteristics of six patients who had antituberculosis drugs as culprit drugs.
| Number of patients | Age/Sex | Disease | Drugs |
|---|---|---|---|
| 1 | 71/F | Urethral tuberculosis | Isoniazid, rifampin, ethambutol |
| 2 | 86/F | Pulmonary tuberculosis | Ethambutol, pyrazinamide |
| 3 | 78/F | Pulmonary tuberculosis | isoniazid |
| 4 | 45/F | Pulmonary tuberculosis | Isoniazid, rifampin, pyrazinamide |
| 5 | 69/F | Pulmonary tuberculosis | Rifampin, ethambutol |
| 6 | 74/F | Pulmonary tuberculosis | Rifampin, ethambutol |
F, female; M, male.
Clinical characteristics of four patients who had undetermined drugs, and three with other culprit drugs.
| Number of patients | Age/Sex | Disease | Drugs |
|---|---|---|---|
| 1 | 65/F | Pneumonia | Ceftriaxone, ibuprofen |
| 2 | 43/M | Bronchitis | Ceftezol, dicofenac |
| 3 | 70/F | Pneumonia | Cefixime, diclofenac |
| 4 | 68/M | Pneumonia | Ceftriaxone, phenytoin |
| 5 | 66/F | Colon cancer | 5-fluorouracil |
| 6 | 38/M | Leukaemia | Desferasiroxa |
| 7 | 61/F | Peptic ulcer | Rabeprazolb |
Numbers 1–4 include patients with undetermined causative drugs.
F, female; M, male.
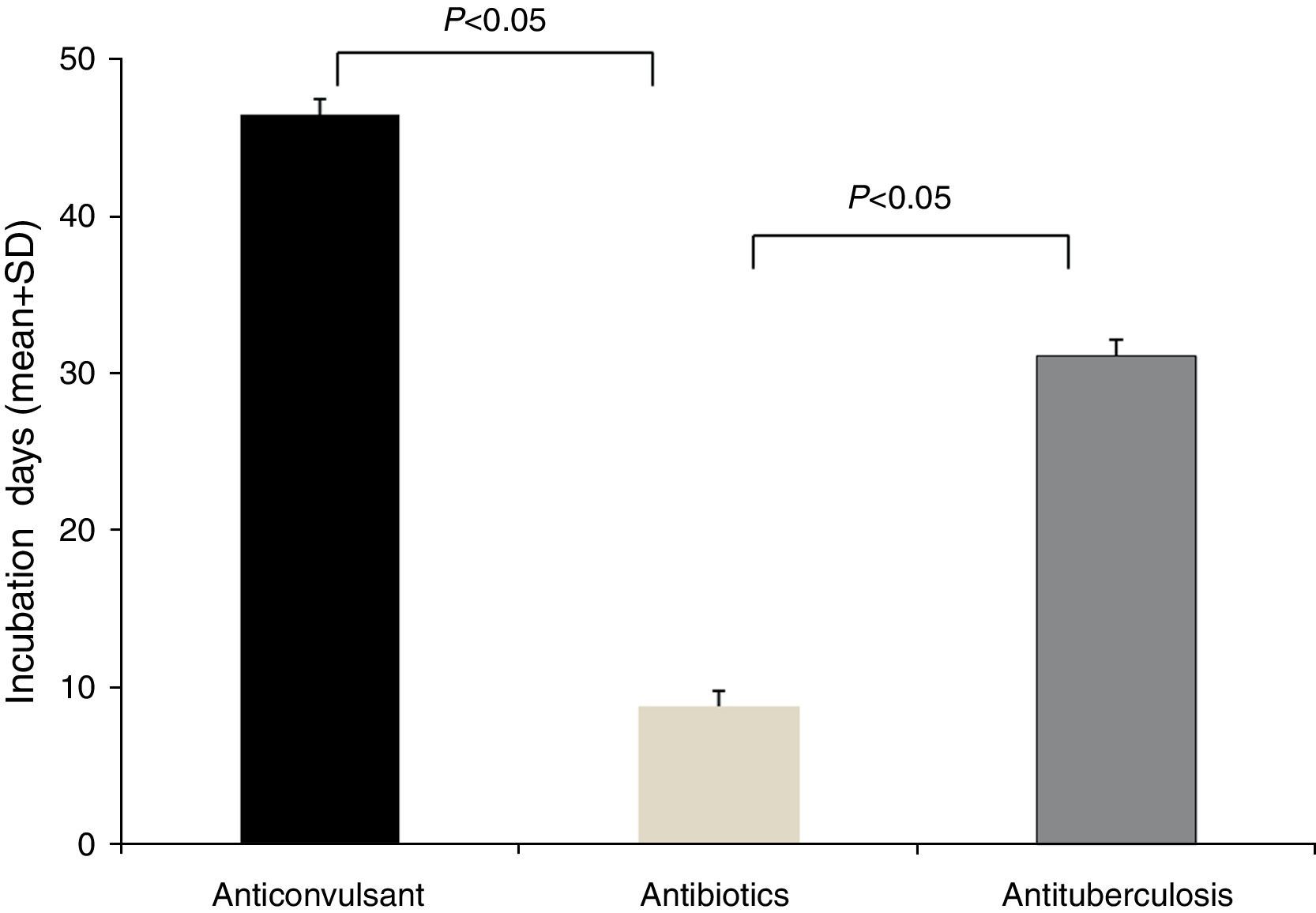
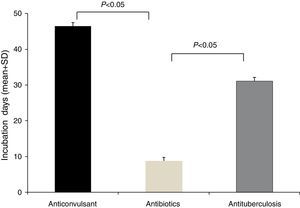
The latency period between the administration of causative drugs and the development of the manifestation of HSS/DRESS ranged from 2 to 120 days, with a mean of 20.2±24.3 days (Table 1). The antituberculosis group had the longest latency period, 46.5±29.9 days (range, 24–100 days), followed by anticonvulsants with 31.1±31.2 days (range, 5–120 days). The latency period for antibiotics was 7.8±8.0 days (range, 2–28 days). A statistical significance was noted between anticonvulsants and antibiotics, as well as antituberculosis and antibiotics (p<0.05, respectively) (Fig. 2).
Laboratory resultsHaematological abnormalities were found to include eosinophilia in 35 patients (77.8%), in addition to atypical lymphocytosis (n=16, 35.6%) and thrombocytopenia (n=7, 15.6%). All patients had involvement of at least one internal organ. Liver was the most frequently involved organ (n=39, 86.7%), followed by kidney (n=8, 17.8%), lung (n=4, 8.9%) and CNS (n=1, 2.3%) (Table 1). None of the patients had positive findings for autoimmune antibodies or IgM antibodies to viruses.
Treatment outcomeThirty-five patients (77.8%) were treated conservatively with topical steroids and antihistamines, while 10 patients (22.2%) were treated with SCS. The mean±SD time of admission in patients with SCS was 46.0±30.1 days (range, 11–111 days), and 18.4±11.7 days (range, 3–54 days) in patients with conservative treatment. Statistically significant differences were noted between the two groups (p<0.05). Most of the patients showed good clinical outcomes. Forty-three patients (95.6%) recovered completely. However, one patient died due to sepsis arising from long-term high-dose SCS treatment, and another patient had progressive hepatic failure, and was referred to another hospital in a remote region.
DiscussionThis study is a retrospective analysis of prospectively collected data of 45 patients with HSS/DRESS in a tertiary referral hospital over a period of two years. The prevalence of HSS/DRESS was more common than had been generally recognised, and the causative drugs were quite variable. In particular, antibiotics and NSAIDs as well as antituberculosis drugs appeared to be major causes of HSS/DRESS. Antibiotics showed relatively short incubation periods (less than two weeks), and seemingly better clinical courses. Most of the study subjects (95.6%) showed better clinical outcomes than had been expected.
The estimated incidence of HSS/DRESS was 1 in 1000–10,000 drug exposures.23 In a referral centre hospital, the prevalence was reported approximately as 6–8 patients per year.(9,16,17,19) The incidence of HSS/DRESS in this study was relatively high, at 45 patients during a two-year period in one tertiary referral centre. We believe that several factors have contributed to our findings. First, we implemented an adverse drug reaction surveillance system in our hospital, by which anyone involved in patient care was able to report any adverse drug reaction through a computerised communication system to a surveillance centre. Two medical doctors and one pharmacist evaluated adverse drug reactions and attempted to identify the causative drugs with an authorised algorithm. Second, any adverse drug reactions were usually followed up for some time until the patient showed an improvement. Some reactions were initially regarded as simple exanthematous drug eruptions, but conditions that progressed were ultimately diagnosed as HSS/DRESS, especially among the antibiotics group. Other reactions were defined initially as simple drug eruptions by doctors who were not familiar with HSS/DRESS, but were ultimately defined as HSS/DRESS. Finally, these data were collected from a tertiary referral centre located in the south-eastern part of South Korea, serving a region of more than three million inhabitants. More than 50% of study subjects were referred from primary or secondary care hospitals. These factors may explain the higher incidence in this study compared to previously published studies.9,16,17,19
One prominent result of this study was the finding that the most common causative drug group of HSS/DRESS was antibiotics, recognised as the causative agent for 13 patients (28.9%). This figure was relatively higher than a recent retrospective study which identified antibiotics as being the causative agent in 6 of 60 patients (10%).19 This result may have originated from the misconception that HSS/DRESS could develop after the administration of anticonvulsants and allopurinol, and that HSS/DRESS caused by antibiotics showed a relatively milder course than any other causative drugs. Furthermore, incorrect diagnosis as a simple drug eruption by doctors who were not familiar with HSS/DRESS have also likely played a role in prior studies which have observed lower numbers of HSS/DRESS due to antibiotics.
We think traditional culprit drugs, for example anticonvulsants and allopurinol, have higher risk than other drugs. However, our results also postulated that non-traditional causative drugs, for example antibiotics, antituberculosis and NSAIDs can cause HSS/DRESS. More attention should be paid to these non-traditional causative drugs, as new causative drugs of HSS/DRESS.
A delayed onset of symptoms by 2–6 weeks after the initiation of the causative drug is a feature of HSS/DRESS.3 In this study, the incubation time of the antibiotics group showed a significantly shorter duration than that of the anticonvulsants or antituberculosis group (Fig. 2). This phenomenon can be found in other reports of HSS/DRESS caused by antibiotics.9–14 We cannot explain this finding accurately, but it is likely that this occurs due to different drug metabolisms and variable individual sensitivity to metabolites of antibiotics. It is possible that a long latency period is adequate for some drugs, especially anticonvulsants. We believe that latency periods may be variable according to each culprit drug.
At present, HSS/DRESS is generally treated with moderate- or high-dose SCS, but response may be suboptimal and can result in a prolonged exposure to SCS.18 A recent retrospective analysis showed that 75% of study subjects were treated with SCS, and that the death rate was 10%.19 In this study, SCS was administered to only 10 patients (22.0%), and the remaining 35 patients (77.8%) were treated conservatively without SCS. Furthermore, the proportion of complete recovery was 95.6%, and seemed higher than that of previous reports. A significant difference was noted in the number of admission days of SCS treated and conservatively treated group. However, the data were insufficient for direct comparison with previous published results, because there was a discrepancy in the composition of study patients, as well as in the culprit drugs.
This study has several limitations. First, the culprit drugs were determined based on subjective clinical decisions, and not with objective methods such as lymphocyte toxicity assay or LTT. Second, evaluations to find out the association between viral reactivation and HSS/DRESS development should have been performed but were not.
In conclusion, drugs associated with HSS/DRESS were variable, and attention should be paid to many new causative drugs, especially antibiotics and NSAIDs. HSS/DRESS was not more uncommon than generally recognised, and most patients with this disease showed better clinical outcomes than might have been generally expected based on previous findings.
Ethical disclosuresPatients’ data protectionConfidentiality of data. The authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals for this investigation.
Conflict of interestAll authors have no conflicts of interest in this study. The authors alone are responsible for content and the writing of the paper.
This work was supported by Dong-A University research fund.