Ciclesonide (CIC) is an effective inhaled corticosteroid for treating asthmatic children. However, its effect on airway inflammation assessed by the fraction of exhaled nitric oxide (FENO) in children with persistent asthma is virtually unknown. We aimed to assess the effect of once-daily generic CIC, 80 or 160μg, on FENO, lung function, asthma control and bronchial hyperresponsiveness, in atopic children with persistent asthma.
MethodsThis was a 12-week, randomised, double-blind, parallel-group study. Sixty children with mild-to-moderate persistent asthma were recruited. Changes in FENO, asthma control score, lung function (FEV1) and bronchial hyperresponsiveness to methacholine (BHR) were used to assess the effects of both CIC doses. Non-normally distributed variables were log-transformed to approximate normality, and parametric tests were used for comparisons within and between groups at baseline and after 12 weeks of treatment.
ResultsIn the CIC 80μg group, FENO decreased from 45.0ppb (95% CI 37.8–53.7) to 32.7ppb (95% CI 21.0–47.3) at the end of study (P=0.021), whereas in the CIC 160μg group, FENO decreased from 47.3ppb (95% CI 40.4–55.3) to 30.5ppb (95% CI 24.1–38.7) (P<0.001). The difference between groups in FENO at the end of study was not significant (P=0.693). There was a significant improvement of asthma control with both CIC doses but there was no significant change in BHR or FEV1 in either group.
ConclusionOnce-daily generic ciclesonide (80μg or 160μg), for 12 weeks, is effective to improve airway inflammation and asthma control in atopic children with persistent asthma.
Inhaled corticosteroids (ICSs) are widely recommended as the first-line anti-inflammatory medications for paediatric and adult patients with persistent asthma. Although the effect of different ICSs on asthmatic airway inflammation has been demonstrated in adults, there is much less information regarding childhood asthma, most likely because the invasive methods used in adults to assess the effect of ICSs on airway inflammation are restricted for ethical reasons in children.
FENO is a non-invasive marker of airway inflammation, and it provides useful complementary information for the diagnosis and monitoring of asthma in children.1–3 Together with other lung function tests, FENO has been employed to evaluate the effects of conventional ICSs such as beclomethasone, budesonide and fluticasone,4–6 and also of extra-fine corticosteroid aerosols (mass median aerodynamic diameter of ≤1.2μm) such as HFA-beclomethasone and ciclesonide.7,8
Ciclesonide (CIC) is safe and effective for improving asthma symptoms, lung function and BHR in asthmatic children, with apparently undetectable systemic effects.9–14 Although conventional ICSs are effective at reducing airway inflammation as assessed by FENO in asthmatic children,4–7 there is little information about the effect of CIC on airway inflammation in paediatric patients. The available evidence comes from studies mainly involving adults.8,15
The present study was undertaken to determine the effect of once-daily generic ciclesonide, 80μg or 160μg, for 12 weeks on the level of FENO, asthma control, lung function and airway responsiveness to methacholine in atopic children with mild-moderate persistent asthma.
MethodsThis was a randomised, double-blind, and parallel-group study carried out during the year 2013 at the Hospital El Pino, Santiago, Chile. Sixty children (aged 7–15 years) with mild-to-moderate persistent asthma, positive prick test to one or more common aeroallergens, FENO>25 parts per billion (ppb) and regular treatment with budesonide or fluticasone during the previous 3 months participated in this study. After a 1-week run-in period when children received the ICS as prescribed at their primary care health centres, they were randomly allocated to receive generic CIC (Disbronc, Neumobiotics, CIPLA) one puff of 80 or 160μg once daily for 12 weeks, with salbutamol as rescue medication. All aerosols were inhaled using a plastic spacer treated with detergent. The devices containing CIC 80 or 160μg per actuation were indistinguishable from each other and were numbered according to randomisation; patients, parents and study personnel were blinded until finishing the study.
FENO measurements and asthma control assessments were performed every 30 days. Spirometry and methacholine bronchial challenge were performed at baseline and after 12 weeks of treatment. Tests were carried out on two consecutive days in the same order (first FENO, then spirometry and methacholine); salbutamol was discontinued for 12h before testing, and ICSs were maintained according to prescription. Participating children were not using long-acting beta-2 agonists, oral corticosteroids, anti-histamines, antileukotrienes or theophylline. The primary variable was the change in mean FENO from baseline to the end of the study. Secondary variables were changes in the Asthma Control Test (ACT) score, FEV1 and BHR to methacholine after 12 weeks of treatment.
On-line single breath FENO measurements (NIOX MINO, Aerocrine AB, Solna, Sweden) were performed according to the ATS guidelines for FENO interpretation.1 Children were asked to inhale to total lung capacity through the mouthpiece connected to the FENO device and then to exhale for 10s at 50mL/s, assisted by visual and auditory cues provided by the device.
Spirometry was performed using a pre-Vent flow sensor with the Medgraphics CPFS/D processing system (Medical Graphics Corp.; St. Paul, MN, USA). The percentage of predicted value for each parameter was calculated according to Knudson's equations.16 Methacholine bronchial challenge was performed if the FEV1 was≥80% of the predicted value using a modified Cockcroft's method.17
A skin prick test for eight common inhalant allergens was performed on the forearm, as was a positive (histamine) and a negative (solvent) control. The following allergens were employed: Dermatophagoides pteronyssinus, Dermatophagoides farinae, cat, dog, Alternaria, a grass mixture, a tree mixture and a weed mixture (Nelco Laboratories, NY, USA). Atopy was defined as a positive reaction (wheal size measuring 3mm or more after subtraction of the control value) to one or more allergens.
Asthma control was evaluated using the ACT.18 The questionnaires for children aged <12 and ≥12 years were filled in by their parents or the children themselves, respectively, during the medical interview, at baseline and every 30 days until the end of study. Physicians were allowed to clarify parents’ and children's doubts as to the meaning of questions. Patients with a score ≤19 were considered to have uncontrolled asthma.
The systemic effect of both CIC doses was assessed by measuring cortisol in 24-h urine samples at randomisation and the end of the study; urinary free cortisol was determined by radioimmunoassay with a reference range of 5–50μg/24h. Fungal culture of the oro-pharynx was performed in all patients at baseline and at the end of the study for eventual candidiasis induced by inhaled CIC. Height was measured by stadiometry.
This study was approved by the Scientific Ethics Committee, Chilean Ministry of Health, Southern Metropolitan Area of Santiago, Chile. Full informed and signed consent was obtained from all parents.
Statistical analysisFENO and all positively skewed variables were log-transformed to approximate normality. Parametric tests (independent and paired samples) were used for comparisons between and within groups, at baseline and at the end of the study; the results of log-converted variables are presented as back-transformed values (i.e., geometric means and 95% CIs). Data were analysed using statistical software (SPSS 15.0, Chicago, USA, and MedCalc 13.2, Ostend, Belgium) and P<0.05 was considered statistically significant. Repeated-measures ANOVA was employed to compare between-group measurements of FENO and ACT that were assessed every 30 days; associations between FENO, PC20, lung function, and ACT at entry were assessed by linear regression. A difference of ≥1 doubling dilution (DD) between baseline and the end of the study was considered a significant reduction of BHR to methacholine. The DD difference was calculated as the log10PC20 difference between entry and the end of the study divided by log10 2. A reduction of at least 20% in FENO for values over 50ppb or more than 10ppb for values lower than 50ppb1 was considered an estimate of a significant response to CIC. The proportion of children in each group (CIC 80 or 160μg) who had a significant improvement in FENO, ACT score and BHR to methacholine after 12 weeks of treatment were compared using the chi-squared test with Yates's correction for continuity (two-tailed). A power calculation determined 29 patients completing would ensure 85% power (two-tailed, α error=0.05) to detect a mean difference of 12ppb in FENO between treatments (SD=15ppb).
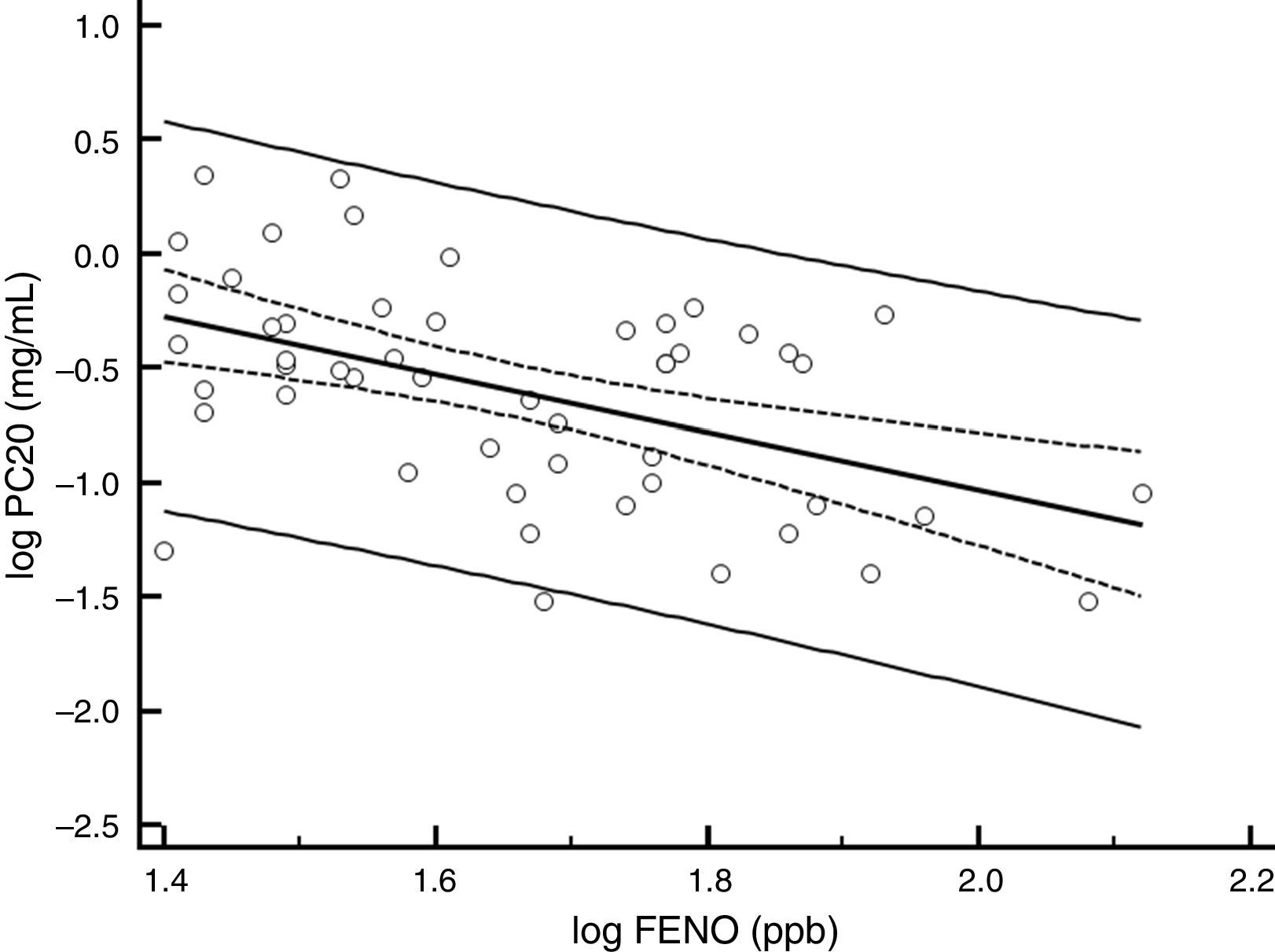
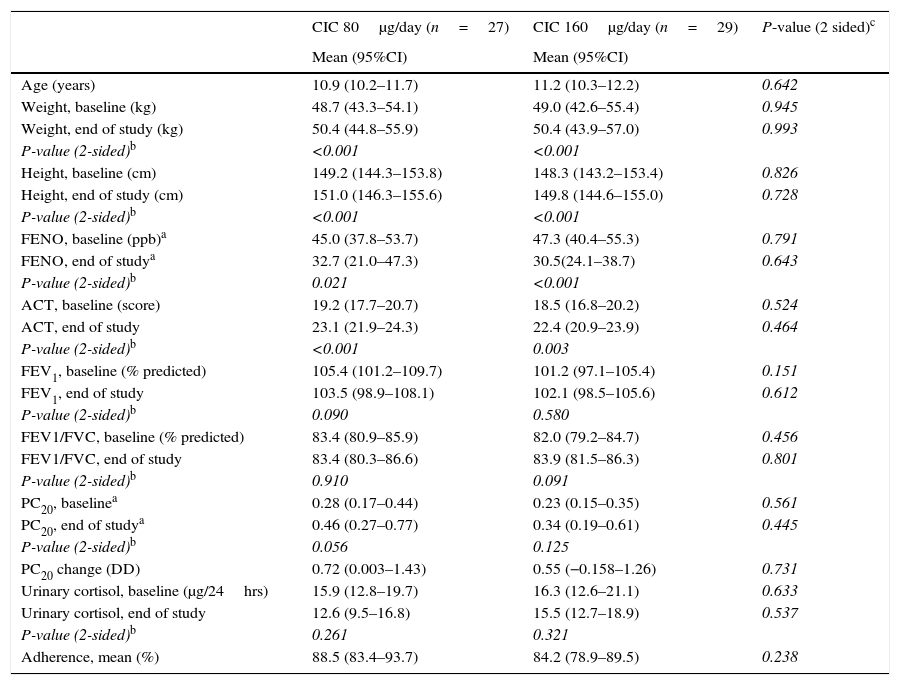
ResultsBaselineOf the 60 children who entered the study, 56 completed all visits and measurements: 27 (15 boys) in the CIC 80μg group and 29 (17 boys) in the CIC 160μg group (Table 1). Four children were withdrawn from the study: two because of asthma exacerbation requiring oral corticosteroids (one in each group) and two in the CIC 80μg group because they or their parents were unwilling to continue with study visits and procedures. There was no significant difference between groups (CIC 80 and 160μg) at baseline or at the end of the study regarding age, height, weight, FENO, methacholine PC20, ACT score, FEV1, or 24-h urinary free cortisol (Table 1). At baseline, FENO was inversely and significantly related with PC20 methacholine (P<0.001), (Fig. 1) and VEF1/FVC (P=0.036), whereas PC20 was directly correlated with VEF1/FVC (P=0.020).
Mean values (95% CI) at baseline and after 12 weeks of treatment with once-daily CIC (80 or 160μg) in children with persistent asthma.
| CIC 80μg/day (n=27) | CIC 160μg/day (n=29) | P-value (2 sided)c | |
|---|---|---|---|
| Mean (95%CI) | Mean (95%CI) | ||
| Age (years) | 10.9 (10.2–11.7) | 11.2 (10.3–12.2) | 0.642 |
| Weight, baseline (kg) | 48.7 (43.3–54.1) | 49.0 (42.6–55.4) | 0.945 |
| Weight, end of study (kg) | 50.4 (44.8–55.9) | 50.4 (43.9–57.0) | 0.993 |
| P-value (2-sided)b | <0.001 | <0.001 | |
| Height, baseline (cm) | 149.2 (144.3–153.8) | 148.3 (143.2–153.4) | 0.826 |
| Height, end of study (cm) | 151.0 (146.3–155.6) | 149.8 (144.6–155.0) | 0.728 |
| P-value (2-sided)b | <0.001 | <0.001 | |
| FENO, baseline (ppb)a | 45.0 (37.8–53.7) | 47.3 (40.4–55.3) | 0.791 |
| FENO, end of studya | 32.7 (21.0–47.3) | 30.5(24.1–38.7) | 0.643 |
| P-value (2-sided)b | 0.021 | <0.001 | |
| ACT, baseline (score) | 19.2 (17.7–20.7) | 18.5 (16.8–20.2) | 0.524 |
| ACT, end of study | 23.1 (21.9–24.3) | 22.4 (20.9–23.9) | 0.464 |
| P-value (2-sided)b | <0.001 | 0.003 | |
| FEV1, baseline (% predicted) | 105.4 (101.2–109.7) | 101.2 (97.1–105.4) | 0.151 |
| FEV1, end of study | 103.5 (98.9–108.1) | 102.1 (98.5–105.6) | 0.612 |
| P-value (2-sided)b | 0.090 | 0.580 | |
| FEV1/FVC, baseline (% predicted) | 83.4 (80.9–85.9) | 82.0 (79.2–84.7) | 0.456 |
| FEV1/FVC, end of study | 83.4 (80.3–86.6) | 83.9 (81.5–86.3) | 0.801 |
| P-value (2-sided)b | 0.910 | 0.091 | |
| PC20, baselinea | 0.28 (0.17–0.44) | 0.23 (0.15–0.35) | 0.561 |
| PC20, end of studya | 0.46 (0.27–0.77) | 0.34 (0.19–0.61) | 0.445 |
| P-value (2-sided)b | 0.056 | 0.125 | |
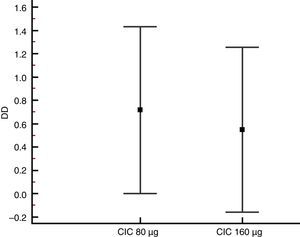
| PC20 change (DD) | 0.72 (0.003–1.43) | 0.55 (−0.158–1.26) | 0.731 |
| Urinary cortisol, baseline (μg/24hrs) | 15.9 (12.8–19.7) | 16.3 (12.6–21.1) | 0.633 |
| Urinary cortisol, end of study | 12.6 (9.5–16.8) | 15.5 (12.7–18.9) | 0.537 |
| P-value (2-sided)b | 0.261 | 0.321 | |
| Adherence, mean (%) | 88.5 (83.4–93.7) | 84.2 (78.9–89.5) | 0.238 |
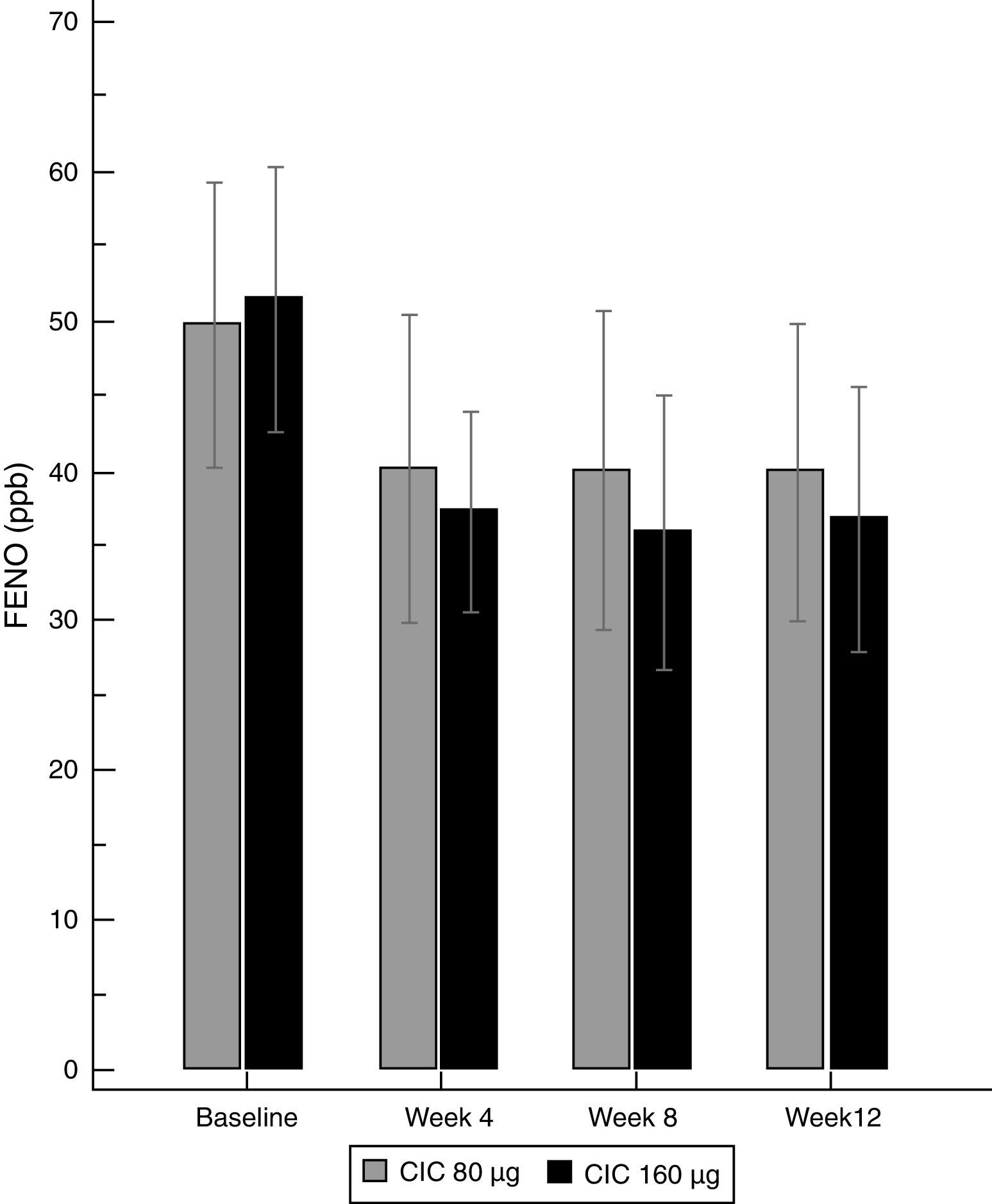
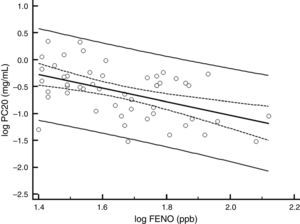
There was a significant decrease in the geometric-mean FENO level in both groups between baseline and endpoint. FENO in the group treated with CIC 80μg decreased from 45.0 to 32.7ppb at the end of the study (P=0.021), whereas in the CIC 160μg group, FENO decreased from 47.3 to 30.5ppb (P≤0.001); see Table 1 for 95% CIs. Both CIC groups showed a significant FENO decrease after four weeks of treatment without further significant changes in measurements at weeks 8 and 12 of treatment (Fig. 2). There was no significant difference between groups in the proportion of children who showed a significant decrease in FENO after 12 weeks of treatment (P=0.633) and the corresponding percentage for CIC80μg and CIC160μg was 59.3 and 69.0%, respectively.
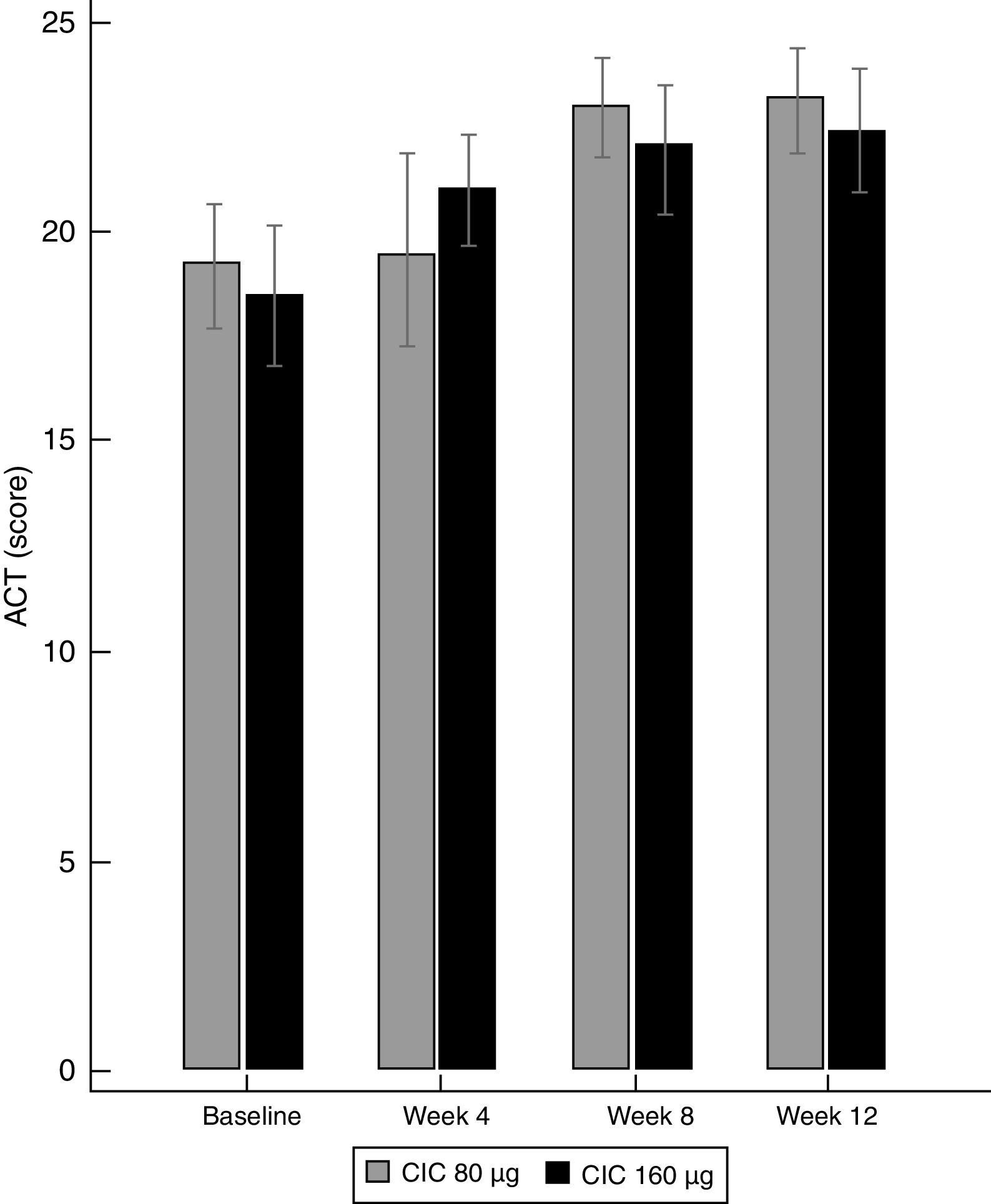
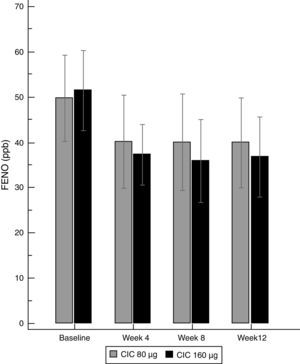
Asthma controlBoth doses of CIC significantly improved the level of asthma control after 12 weeks of treatment. The ACT score increased from 19.2 to 23.1 in the group treated with CIC 80μg (P≤0.001) and from 18.5 to 22.4 in the CIC 160μg group (P=0.003). There was no significant difference in ACT between groups at baseline or after 12 weeks of treatment (Table 1). The increase in the ACT score was significant at week 8, with no further significant changes until the end of the study in both treatment groups (Fig. 3). The difference in the proportion of children who had controlled asthma (ACT score 20 or more) at baseline between the CIC 80μg group (48.1%) and the CIC 160μg group (41.4%) was not significant (P=0.814). After 12 weeks, 85.2 and 86.2%, in the groups treated with CIC 80 or 160μg groups, respectively, had controlled asthma, and the difference between groups was not significant (P=0.783).
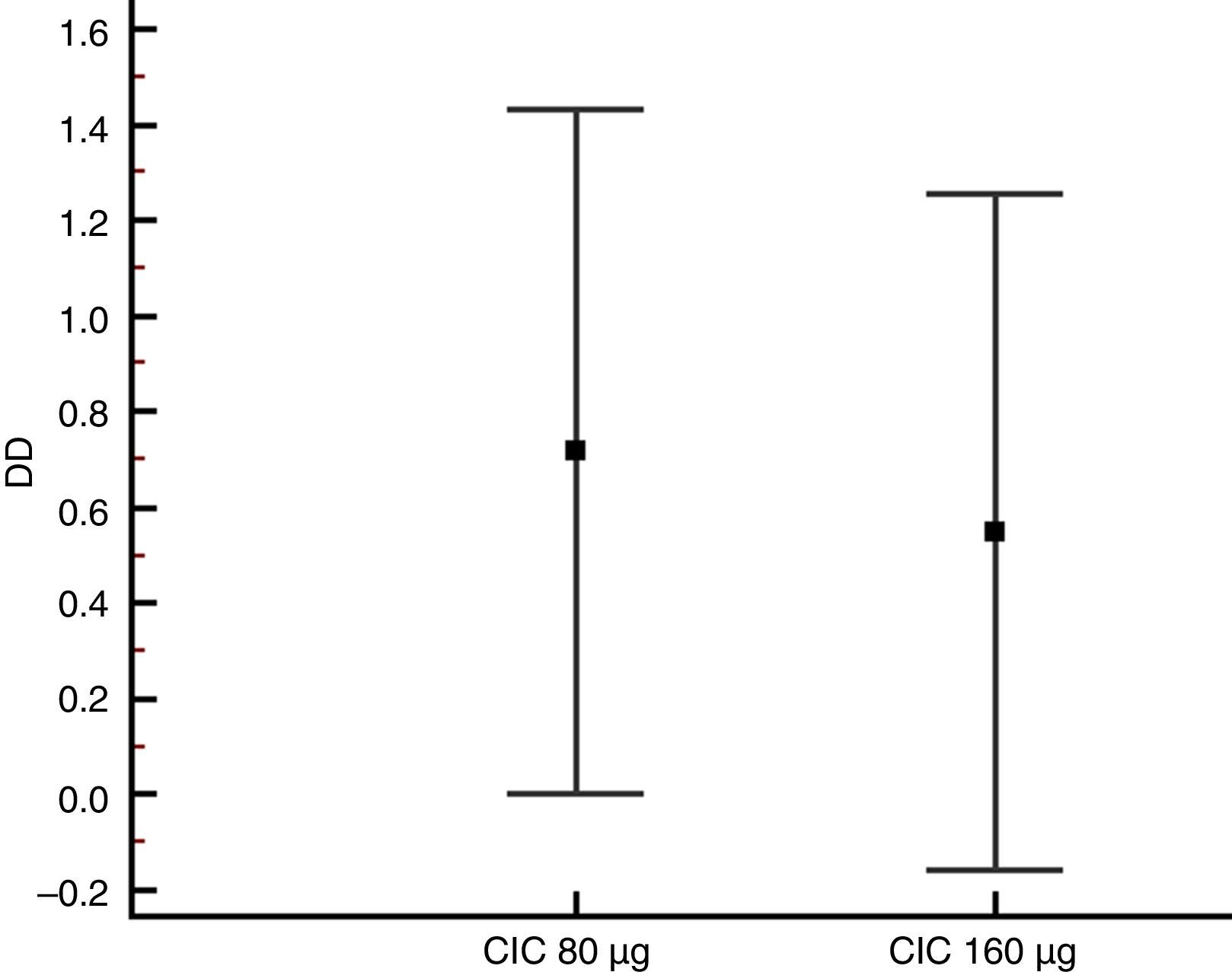
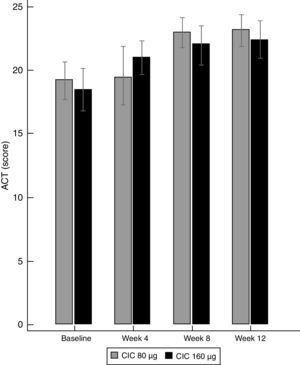
Lung function and BHRThere were no significant differences between groups in terms of FVC, FEV1, FEF25–75% or VEF1/FVC at baseline or the end of the study (Table 1), and no significant change in FEV1, FEF25–75% or VEF1/FVC was found within either group after 12 weeks of treatment. However, FVC showed a significant decrease only in the CIC 80μg group (P=0.012), (Table 1). The effect of both CIC doses on methacholine PC20 was widely variable, with a mean DD change of 0.72 and 0.55 in the CIC 80μg and 160μg groups, respectively (Fig. 4); the two groups had similar magnitudes of DD change (P=0.731). The difference in the proportion of children who had a DD change ≥1 between the 80μg (50.0%) and 160μg groups (32.1%) was not significant (P=0.304). The geometric-mean PC20 for the CIC 80μg group was 0.28mg/mL at baseline versus 0.46mg/mL at the end of the study but the difference did not reach statistical significance (P=0.056); in the group treated with CIC 160μg the difference in PC20 after 3 months of treatment (0.23mg/mL vs. 0.34mg/mL) was not significant (P=0.125).
There was no significant difference in 24-h urinary free cortisol between groups at baseline (P=0.633) or after 12 weeks (P=0.537), and there was no significant change within either group after 12 weeks of treatment. Weight and height showed a significant increase in both CIC groups (Table 1), and the difference between groups at the end of the study was not significant: weight (P=0.993), height (P=0.728). The mean adherence to treatment with both CIC doses was ≥80%, with no significant difference between groups (P=0.238), (Table 1). Once-daily CIC at the employed doses was well tolerated, and none of the patients or their parents reported trouble related to the inhaled medication. At the end of the study, none of the patients had positive oro-pharyngeal cultures for candidiasis.
DiscussionThis study shows that once-daily generic CIC either in a dose of 80μg or 160μg for 12 weeks is effective at decreasing airway inflammation and improving asthma control in atopic children with mild-moderate persistent asthma. To our knowledge, this is the first study assessing the short-term effect of a generic CIC on FENO in asthmatic children.
FENO is a recognised marker of airway eosinophilic inflammation and is useful in asthma management for adjusting ICS doses, monitoring the effect of ICSs, verifying adherence to treatment and determining potential responsiveness to ICSs, among others.1,19–25 The efficacy of different ICSs, including CIC, in decreasing FENO in asthmatic adults and adolescents aged >12years has been documented and occurs as early as one week after starting treatment.21–25
The present study did not find a dose-dependent effect of CIC 80μg or 160μg on FENO, ACT, lung function or BHR to methacholine. Other authors using original CIC in children with persistent asthma have found a significant dose-response effect between 80μg and 160μg for exacerbations and lung function, but not on other outcomes such as BHR to methacholine.12–14 An explanation for this lack of a consistent dose-response effect with CIC may be that ICS dose-response curves tend to be flat, with minor differences in clinical and functional results between low and high doses, as shown by evidence-based analysis.26 In our study, less than half the patients in both CIC groups improved BHR (DD change ≥1) after 12 weeks of treatment. Another study14 using original CIC with doses and time spans similar to our study found a significant improvement of BHR to methacholine. However, other authors found that a significant BHR improvement in asthmatic children treated with budesonide was achieved after 4 months of treatment.27 Thus, the time to reach a significant effect on decreasing BHR varies depending on factors related to treatment, patients, or disease characteristics, among others.28–30 We have previously found a high proportion of BHR to methacholine in children with current asthma symptoms and also in non-asthmatics, which was unrelated to atopy or lung function,17 suggesting that environmental factors could increase airway responsiveness not only in asymptomatic asthmatics but also in healthy individuals. Additionally, the lack of a rapid and significant improvement in PC20 after 12 weeks, as occurred in this study, might also be related to potential pharmacological differences between generic and original CIC, but this remains to be demonstrated by further research.
In the present study, FENO showed a strong association with methacholine PC20 at baseline but not with ACT or FEV1. This finding agrees with other studies showing poor agreement among FENO, symptoms and lung-function measures in asthmatic adults and children.31,32 Concordantly, FENO and BHR are more directly related to inflammatory changes in the bronchial mucosa of asthmatic patients than to symptoms or lung function.33,34
CIC is effective at improving asthma control in asthmatic children.12,35 The present study, using the ACT questionnaires, found that both CIC doses (80 and 160μg) produced a similar and significant improvement in asthma control after 12 weeks of treatment. Despite potential limitations of the questionnaires for assessing asthma control in asthmatic children,36 the use of validated questionnaires for this purpose is strongly recommended by all major asthma guidelines. In addition, ACT is a better instrument to identify uncontrolled asthma in children than lung function,37 with the advantage of being an easily accessible and applicable clinical instrument.
Adherence to treatment is recognised as a crucial element of asthma management and evaluation of treatment efficacy. In asthmatic children, low adherence to ICS treatment results in poor asthma control.38 In this study, the adherence to CIC was good (>80%) in both treatment groups. It is likely that regular assessment of adherence to treatment and the education provided to children and parents on the importance of accomplishing the treatments improved the compliance in the present study.
This study has several limitations that are inherent to short-term studies on ICSs. Our study does not allow for predicting long-term adverse events, assessing the variability of therapeutic responses for the different measurements of efficacy, or evaluating the clinical and functional variations determined by seasonal effects (viruses, pollen, indoor and outdoor pollution). Additionally, short-term studies are unable to examine the expected decline of compliance to study medications observed in long-term treatments and the resulting effects on study outcomes.39,40 However, the present study, involving a well-characterised group of atopic children with mild-moderate persistent asthma, provides evidence on the effectiveness of generic CIC at decreasing inflammation and improving asthma control in those patients. We did not find a significant difference between the effects of CIC 80 and 160μg on FENO, lung function, asthma control or BHR to methacholine. This could be explained at least in part by the relative flatness of ICS dose-response curves. It might also be related to an insufficient sample size, although other studies that used CIC 80 or 160μg and which involved several hundred asthmatic children have not found consistent differences in the effects of both doses on study measurements.12–14
It has been shown that CIC is at least as effective as other ICSs for the treatment of asthmatic children, but it does not decrease cortisol excretion.12 However, the scarcity of data and the important methodological differences among studies make it difficult to draw valid conclusions from comparisons of CIC with other ICSs.40 Nevertheless, its special pharmacological characteristics (pro-drug, small-particle aerosol, once-daily inhalation, and safety) make CIC an attractive option for the treatment of paediatric asthma; in the case of generic CIC, its lower cost may represent an economic advantage for parents or health institutions.
ConclusionsGeneric ciclesonide (80 and 160μg) inhaled once daily for 12 weeks improved airway inflammation and asthma control in atopic children with persistent asthma.
FundingThe study was supported by the Vice Presidency of Research, Development and Innovation, University of Santiago de Chile (USACH), and Hospital CRS El Pino, Santiago, Chile.
Author contributionsAll authors participated in the study conception and design; data collection, analysis, and interpretation; manuscript drafting and revision; and approval of the final manuscript.
Ethical disclosuresProtection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors have no conflicts of interest to declare. The authors alone are responsible for the content and writing of the paper.
The authors thank all the children who participated in the study and their parents. We thank to Elba Soto, Isabel Bacigalupo and Sara Valenzuela for their valuable paramedical collaboration in this study.