Eosinophilic oesophagitis (EO) is an infrequent disorder that is currently underdiagnosed. It has been described in both adults and in children, and is more prevalent among males. The etiology of EO is not clear, though atopy has been suggested as playing an important role in the development of the disease.
The clinical presentation of EO is varied, and a differential diagnosis with other digestive tract disorders is required — particularly gastro-oesophageal reflux. Dysphagia and food bolus impactation within the oesophagus are the most characteristic symptoms. Diagnostic confirmation is obtained from multiple oesophageal biopsy, with the detection in some sample or samples of over 15 eosinophils per high-magnification microscopic field.
An allergological study is needed to evaluate the existence of allergens (perennial or seasonal environmental allergens and food allergens) responsible for the eosinophilic infiltration found at oesophageal level.
There is no specific treatment for EO, and topical corticosteroids (swallowed) are currently the pharmacological treatment of choice. Dietary therapy in children with food allergy as the causal factor may prove effective, though the existence of polysensitisation complicates the correct implementation of such treatment. Oesophageal dilatation is reserved for cases with severe dysphagia, and is not without complications. Treatment with anti-IL-5, antileukotrienes, azathioprine, 6-mercaptopurine, anti-IgE, etc., could constitute alternatives to topical corticosteroids, although information is still lacking on their long-term safety and efficacy in the paediatric population.
The first report of eosinophilic inflammation at oesophageal level was made by Dobbins in 1977. This in turn was followed by the description of patients resistant to proton pump inhibitor treatment for possible gastro-oesophageal reflux who presented eosinophil infiltration of the oesophageal mucosa. However, it was not until 1993 when eosinophilic oesophagitis (EO) was defined as a clinically differentiated disorder independent of the rest of eosinophilic diseases of the gastrointestinal tract1–3.
A range of pathologies are characterized by eosinophil infiltration of the oesophagus, including EO. The latter disorder is infrequent and is characterized by chronic eosinophilic infiltration of the oesophagus.
Although there are few epidemiological data on the prevalence and incidence of EO, the number of diagnosed cases has increased in recent years – probably due to improved knowledge of the disease. Some information has been published on the prevalence of EO in different settings: 1/1500 in Cincinnati (United States) (paediatric population), 1/40,000 in Switzerland, and 4/1000 in Sweden (adult population)4. It is not uncommon for oesophagitis secondary to gastro-oesophageal reflux to constitute a first diagnostic orientation in children presenting symptoms compatible with reflux, followed by the diagnosis of EO at a later point in time – generally as a consequence of poor patient response to antireflux therapy. According to Markowitz et al., up to 8–10 % of all children initially diagnosed with gastro-oesophageal reflux unresponsive to usual treatment may actually suffer EO5.
Eosinophilic oesophagitis has been described both in children and in adults, and is more prevalent among males1,6,7. Although some studies suggest that the incidence of EO could be higher in Caucasians, there are insufficient data to conclude that there is a racial predisposition to develop the disease8.
ETIOLOGYThe etiology of EO is not clear, though allergy has been suggested as playing an important role in the development of the disease4,9. Different studies found in the literature postulate that allergy could constitute one of the bases of the disease, since high percentages of atopy have been reported in the series published to date (50–81 %), and the presence of an abundant eosinophilic infiltration in the oesophageal mucosa of these patients has been described10,11. The association between EO and a type Th2 immune response appears clear, although recent studies postulate that Th1 helper lymphocytes also participate in the physiopathology of the disease – suggesting a mixed-type immunological disorder12,13 (Table I). The increase in the prevalence of allergic diseases seen in recent years could be one of the causes of the growth in the number of patients diagnosed with EO – thereby supporting the hypothesis that this is an allergic disorder in which the target organ is the oesophagus.
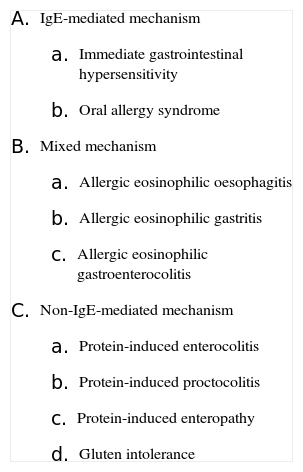
Classification of gastrointestinal food hypersensitivity
|
Both food allergens and aeroallergens appear to be implicated in the etiology of EO. Kelly et al. observed that children given an elemental diet showed clinical and histopathological improvement of EO, following the absence of significant variations in the symptoms with antireflux therapy4,14. There have been reports of variations in the symptoms of EO in patients with seasonal pollen allergy – the aeroallergen being cited as a common etiological agent in both pathologies4,15–17.
There is evidence of a genetic predisposition in EO, specifically at the level of the gene encoding for eotaxin-3. In this context, eotaxin is a chemokine produced by the cells of the endothelium and epithelium, and macrophages of the gastrointestinal tract, including the oesophagus. Alteration in the expression of the gene regulating eotaxin-3 could be responsible for the pathogenesis of EO4,18. Interleukin 5 also has been implicated in the etiopathogenesis of EO, since high IL-5 levels would favour development of the disease18.
CLINICAL MANIFESTATIONSTraditionally, EO has been regarded as a paediatric disease, though in recent years there has been a rise in the number of adult cases diagnosed. Males are affected in up to 75 % of all cases, though some series report even higher percentages1,19. A large proportion of patients have familial and/or personal antecedents of atopy (rhinitis, asthma, food allergy or atopic dermatitis)10.
The clinical presentation is varied, though dysphagia (particularly in response to solid food) and food bolus impactation within the oesophagus are the most characteristic symptoms. In small children the rejection of feeding is the dominant manifestation. Other frequent clinical manifestations are vomiting, regurgitation, growth retardation, epigastric pain, chest pain, abdominal pain or loss of appetite. In many cases the clinical picture can be confused with gastro-oesophageal reflux disease, and EO is probably underdiagnosed because of this. When antireflux treatment proves ineffective, the possibility of EO should be considered. Although EO and gastro-oesophageal reflux generally present independently, both conditions may coexist in some individuals. However, in such situations oesophageal involvement is usually not as important as in patients with primary EO.
In very advanced cases in which the inflammation persists for years, oesophageal stenosis may develop – giving rise to intermittent and painful dysphagia that ultimately may become constant as the disease progresses.
OESOPHAGEAL DISORDERS WITH EOSINOPHILIC INFILTRATIONThe normal oesophageal mucosa does not contain eosinophils – unlike other regions of the digestive tube such as the intestine or stomach. Therefore, in the presence of eosinophils in the oesophageal mucosa, a differential diagnosis must be established, including EO.
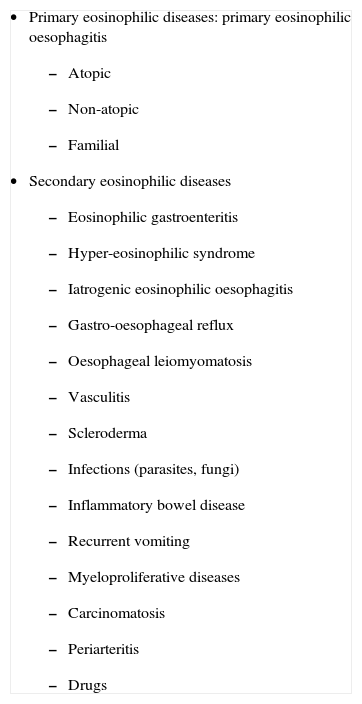
Recently, a new classification of the oesophageal disorders characterized by eosinophilic infiltration has been proposed (Table II)20, allowing a correct differential diagnosis to be established21.
Classification of oesophageal disorders associated to eosinophils
|
Eosinophilic oesophagitis is an underdiagnosed disease, though in recent years there has been a rise in the number of reported cases. It is not uncommon to diagnose EO when a patient initially diagnosed with gastro-oesophageal reflux fails to improve with the usual treatment (dietary measures, antireflux drugs). In some cases the diagnosis of EO has been established following Nissen funduplicature that likewise proves to be ineffective4. The time between the onset of symptoms and the endoscopic diagnosis is generally long in the absence of any clear initial suspicion. A recent study in the paediatric population has found the interval between the onset of symptoms and the diagnosis of EO to be less than one year, in contrast to several years as reported by most studies19.
In the anamnesis it is important to document the presence of personal and/or familial antecedents of atopy (rhinitis, asthma, atopic dermatitis, food allergy), the symptoms of the child, their relationship to food ingestion or exposure to environmental allergens, seasonal variability of the symptoms, their duration, the treatments provided, and patient response to such treatments.
In the study of eosinophilic oesophagitis, allergological assessment is important for determining the presence of sensitisation to allergens and their possible causal correlation to EO. The allergological study should include the following:
- –
Pneumoallergen skin tests (prick tests)
- –
Food skin tests: prick tests, epicutaneous tests22, prick-prick testing with fresh food
- –
Determination of total and specific IgE
- –
Elimination / reintroduction tests
- –
Provocation (challenge) tests
Food allergy has often been associated with EO. In this sense, the most commonly cited foods have been milk, eggs and cereals22. In a recent study published by Plaza-Martin et al. involving a group of 14 children diagnosed with EO, the most frequent food sensitisation corresponded to legumes (57 % of the children) – with a full 93 % of the patients being sensitised to aeroallergens19. The prick-prick test with fresh food is more sensitive than prick testing with the standard extract in the study of food allergy. Some authors have studied delayed responses to food allergens based on epicutaneous tests or patch tests in patients with EO22. Since there are no standardised allergenic extracts for epicutaneous testing with all the potentially implicated foods, such tests are not usually used for the routine assessment of food allergy.
The implication of food allergens in EO has been studied by different authors. In 2002, the group led by Spergel22 published on a series of 26 patients with a mean age of 6.9years diagnosed with EO on the basis of the biopsy findings, and which were subjected to allergological study. Fourteen of the 26 patients were sensitised to environmental allergens as determined by prick testing, and 19 were sensitised to food allergens (epicutaneous testing revealing sensitivity to food allergens in 21 patients). These children were administered a diet lacking those foods to which they were sensitised, with the following results: 18/26 showed total disappearance of the symptoms, and 6/26 showed partial clinical improvement (upon questioning, it was seen that five children had failed to follow the elimination diet correctly). Of the global 26 patients, 24 underwent repeat oesophageal biopsies – with normal findings in 13 cases and histopathological improvement in the remaining 11 subjects.
Seasonal as well as perennial environmental allergens must also be taken into account in patients with EO who report a history of respiratory allergic disease. There have been reports of patients with EO and pollinic respiratory allergy suffering exacerbation of the symptoms of both pathologies during the pollination season – with significant clinical and endoscopic improvement outside this season – and in whom food allergy has been ruled out as a cause of the symptoms15,17,23. The mechanism underlying pollen-induced EO could be similar to that found in EO attributed to food allergens. Patients inhale the aeroallergen, which is deposited in the nasal secretions and is then swallowed – inducing an allergic reaction within the oesophagus in a way similar to food oral allergy syndrome16.
At experimental level, different authors have demonstrated a relationship between the inhalation of fungi and the development of eosinophilic oesophagitis18,24. In the study published by Mishra in 2001, a group of mice were subjected to oral, intranasal and intragastric exposure to Aspergillus fumigatus during three weeks. A significant increase was observed in the number of eosinophils in those mice subjected to intranasal exposure compared with those subjected to oral or intragastric allergen exposure. In the mice exposed to the allergen via the intranasal route, significant differences were observed versus treatment with saline solution, with higher eosinophil counts in both the oesophageal mucosa and in peripheral blood and bronchoalveolar lavage – with no significant variations in the gastric or duodenal mucosa. In the same study, lesser increases in eosinophil count were recorded both in mice with eotaxin deficiency and in those with an absence of IL-5.
The presence of peripheral eosinophilia and elevated plasma total IgE constitute variable findings, and although these parameters are always assessed in the general allergological study, they do not represent diagnostic or prognostic markers for EO. At present, no specific diagnostic or follow-up marker for this disease has been established.
The clinical suspicion of EO must be confirmed by fibroscopy with the obtaining of biopsies from different zones of the oesophagus, since disease involvement may not be homogeneous.
Macroscopically, the oesophageal mucosa may appear normal. However, the most common histological findings are erythema, oedema, loss of the vascular pattern, linear and longitudinal folds, mucosal friability (referred to as “crepe paper oesophagus”)25, a whitish punctate pattern (corresponding to accumulations of eosinophils that protrude within the oesophageal mucosa and which can be mistaken for oesophageal candidiasis)1. In more advanced cases, mucosal thickening can be seen, along with nodules and stenotic rings reminiscent of the tracheal structure (“oesophageal trachealization”)1. These cases are usually characterized by alterations in oesophageal motility.
At histological level, an eosinophilic infiltration predominantly located in the proximal and middle thirds of the oesophagus is the fundamental feature in EO, and the infiltrate may be found in all the layers of the oesophagus. Since eosinophilic infiltration in EO may show a patch-type distribution, the maximum number of eosinophils can vary considerably among the different samples obtained from the same patient.
The normal oesophagus does not contain eosinophils. The presence of ≥ 15 eosinophils/high-magnification microscopic field in the biopsy, in the absence of reflux confirmed by oesophageal pHmetry, allows us to distinguish EO from other processes such as gastro-oesophageal reflux disease (GERD).
It has been suggested that the number and location of eosinophils in the oesophagus could contribute to the differential diagnosis between EO and gastro-oesophageal reflux (Table III)4. Involvement of the distal oesophagus is characteristic of reflux disease. In relation to the number of eosinophils, up to 6 cells per field has been proposed as indicative of reflux disease, while over 20–24 eosinophils per field would be indicative of EO26. This distinction would not be useful in those patients with intermediate eosinophil counts. Recently, the consensus report of the American College of Gastroenterology has established a cut-off eosinophil count for EO of 15 cells per high-magnification microscopic field – this being one of the three diagnostic criteria defined by this association (Table IV)8.
Differences between eosinophilic oesophagitis and gastro-oesophageal reflux
| Location | EO | GER |
| Proximal and middle oesophagus | Distal oesophagus | |
| Number of eosinophils/field | ≥ 15 | < 6 |
| Response to PPI treatment | No improvement | Improvement |
EO: eosinophilic oesophagitis; GER: gastro-oesophageal reflux; PPI: proton pump inhibitor.
In addition to the presence of eosinophils, other histopathological alterations that can be seen in these patients are basal cell hyperplasia, an increase in the number of lymphocytes (both CD4+ and CD8+ T-cells, suggesting the existence of a mixed Th1-Th2 immunological reaction) and mast cells, fibrosis of the lamina propria, and elongation of the vascular papillae.
When eosinophilic infiltration is intense, alterations in oesophageal motility can be observed. A recent study involving paediatric patients with EO has evidenced changes in oesophageal structure similar to those seen in remodelling of patients with bronchial asthma27.
Other necessary explorations in the study of EO are the following:
- –
Monitorisation of oesophageal pH: this allows us to discard the presence of gastro-oesophageal reflux.
- –
Oesophageal transit: this technique is useful particularly in those patients with important involvement of the oesophageal wall, in order to determine the calibre of the oesophagus before endoscopy, and to avoid the risk of oesophageal rupture.
- –
Oesophageal manometry: this allows us to evaluate the existence of alterations in oesophageal motility.
No molecular marker for the diagnosis of EO has been established to date.
TREATMENTThe treatment of EO is complicated due to different reasons: the natural course of the disease is not clear, few comparative studies have been made of the different treatment options, and, moreover few management options are available at the present time.
Treatment should be individualized, and the assessment of efficacy must be based on clinical evaluation of the patient – with the repetition of endoscopy and oesophageal biopsies in order to evidence changes during and after treatment.
The existing therapeutic options for EO include an elimination diet, drug therapy, and oesophageal dilatations21.
Dietary treatmentDiets involving the exclusion or elimination of one or more foods can be introduced after completing a correct allergological study. The aim is to identify a possible causal relationship between the food or environmental allergen to which the patient is sensitised and the presence of eosinophilic infiltration in the oesophagus, responsible for the symptoms of the patient. In order to confirm that EO is due to food allergy, each food should be subsequently reintroduced on an individual basis to confirm that the symptoms are reproduced and eosinophilic infiltration reappears within the oesophagus.
Studies have been made in paediatric populations in which management consisted of an elemental diet with amino acids28. This is an alternative treatment reserved for those cases characterized by multiple food allergy, since nutritional deficiencies may result in the absence of required counselling by a nutritionist. Treatments based on elemental formulas are usually not well tolerated by children.
According to the observations by Spergel et al. in a group of 146 children with EO sensitised to food allergens (confirmed by prick and/or patch testing), avoidance diets appear to be effective. Of the total 146 children administered a diet designed to avoid those foods to which they are sensitised (an elemental diet being decided on in cases of polysensitisation), 112 were classified as clinical and histopathological “responders” (biopsy after treatment with < 5 eosinophils/high-magnification field); 33 as “partial responders”, since both the symptoms and the biopsy findings (12 eosinophils/high-magnification field) showed only partial improvement; and 15 as “non-responders” (36 eosinophils/high-magnification field)29.
Other authors have attempted to introduce restriction diets for foods traditionally considered to be allergenic, without individualising the allergological study or taking into account the dietary habits of the patient or the geographical setting involved – with variable results30. This type of diet, while better tolerated than an elemental diet because it maintains the solids and imposes fewer restrictions on the number of foods, would not be applicable to children with sensitisation to multiple foods, and should not be used empirically without a prior supporting allergological study.
The persistence of symptoms in an allergic patient who correctly follows an elimination diet may be due to different causes: an incorrect etiological diagnosis, the presence of food polysensitisation, sensitisation to aeroallergens, etc.
To date, no diet duration has been established for assessing the clinical and histological response, or the relapse rate following the suppression of treatment.
Drug treatmentThere is no specific pharmacological treatment for EO. Topical corticosteroids have been and remain the most widely used drugs for the treatment of eosinophilic oesophagitis. At present, fluticasone propionate applied to the tongue so that the patient swallows the medication is the treatment of choice in EO. The drug is quickly metabolised, since fluticasone is scantly absorbed within the gastrointestinal tract. The results obtained are similar to those afforded by systemic corticosteroids, though with fewer side effects. As a result, fluticasone propionate is the treatment of choice in both children and in adults. Although there are no data on the frequency of oesophageal candidiasis secondary to such treatment, it does not seem to be a common side effect. Swallowed and inhaled budesonide has also been evaluated, with good results31,32.
It is advisable to use only oral corticosteroid doses for outbreaks of EO, and to reserve topical corticosteroids for maintenance treatment. The recommended systemic methylprednisolone dose is 0.5mg/kg/day during 6months, followed by gradual dose reduction7. Although the histological improvement is greater than with topical corticosteroids, the corresponding side effects rate is also far higher; such systemic dosing is therefore reserved for very concrete cases33. The existence of corticosteroid-resistant patients has recently been reported34.
Treatment with mast cell membrane stabilisers such as sodium cromoglycate has been used, with variable results. The use of ketotifen reduces eosinophil infiltration, peripheral eosinophilia, and improves the symptoms when used for prolonged periods of time (4–6months)1, although these two treatments are currently little used.
Leukotriene antagonists are able to improve the symptoms of EO in adults, though without documented improvement in the degree of eosinophilic infiltration in the oesophagus. It has been seen that montelukast reduces eosinophil activity and even peripheral blood eosinophilia – though it exerts no significant effects in terms of the reduction of eosinophil counts in the oesophageal infiltrate35. Similar cysteinyl-leukotriene levels are found in the oesophageal mucosa of children with EO and healthy children; in this context, further studies on the efficacy of antileukotrienes in the treatment of EO are needed36,37.
Treatment with anti-IgE antibodies may afford anti-inflammatory effects in EO, though information warranting their systematic use is lacking.
Cytokine treatment could play a role in the management of EO. Parenteral administration of the anti-IL-5 monoclonal antibody mepolizumab has yielded good results in short patient series, improving the symptoms and histology in adults. However, paediatric studies versus placebo and other treatments are needed, along with long-term safety and efficacy studies38. In relation to IL-13, experimental studies have been made with anti-IL-1339. Cytokine-based treatments appear to offer a promising future in the management of EO and other diseases characterized by the presence of tissue eosinophil infiltrates, though more studies versus placebo and other treatments are needed, focusing on the paediatric population and adults, in order to gain sufficient clinical experience with such therapies.
Use has been made of azathioprine and 6-mercaptopurine, i.e., drugs widely used in inflammatory bowel disease, in patients with EO – with promising results, particularly in corticosteroid-resistant subjects40.
Endoscopic oesophageal dilatationOesophageal dilatation constitutes symptomatic treatment with a high risk of complications (rupture). The technique affords temporary patient relief, but exerts no effect upon the oesophageal inflammation; it is therefore not considered to be the treatment of choice in patients with EO41. However, some authors regard dilatation as an alternative in patients in whom topical corticosteroid therapy proves ineffective42 – although alternatives to topical corticosteroids are currently being investigated which could avoid the need for such invasive treatment.
CONCLUSIONSEosinophilic oesophagitis (EO) is an infrequent yet underdiagnosed disease. Its precise etiology is not clear, though a mixed Th1-Th2 immunological response may be involved. EO should be suspected in any patient with dysphagia or food impactation, or in those cases in which gastro-oesophageal reflux disease (GERD) is suspected but the usual treatment for GERD proves ineffective. The presence of abundant eosinophilic infiltrates in the upper and middle thirds of the oesophagus is suggestive of EO, although other causes must be discarded.
Little information is available on the course of this disease or on the duration and long-term effects of the different treatment used (pharmacological, dietary, endoscopic).
There is no single definitive treatment option for EO, though swallowed corticoids are presently the management of choice. New treatments with monoclonal antibodies are currently being investigated and may prove effective in application to EO – though there is still insufficient information to warrant their systematic use.