Patients with atopic dermatitis frequently present recurrent infections by pyogenic bacteria or by intracellular microorganisms, suggesting an immune disorder.
ObjectiveLaboratorial investigation of phagocyte activity and chemotactic response by neutrophilic polymorphonuclear and mononuclear phagocytes in the peripheral blood of patients with atopic dermatitis from moderate to severe.
MethodsThrough a transversal study, patients with atopic dermatitis from moderate to severe were selected. The neutrophilic and mononuclear phagocytes were separated and the phagocytic ingestion of zymosan particles was analysed, in addition to migration distance to the bacterial lipopolysaccharide chemotactic factor, comparing the results to the values obtained from healthy individuals within the same age group.
ResultsNineteen patients were selected, 11 female and 8 male. The mean age was 6.47 years (±4.65). Among the 19 patients studied, 14 (73.68%) presented a reduction in the neutrophilic and mononuclear phagocyte activity, with two (1.53%) patients presenting a reduction in the activity of both phagocytes.
ConclusionOur results demonstrated a reduction in chemotactic response and phagocytic activity by neutrophilic and/or mononuclear phagocytes in the majority of patients with atopic dermatitis from moderate to severe. Our results were coherent with the clinical data concerning the higher incidence of infections by pyogenic bacteria and fungi in patients with atopic dermatitis, which are microorganisms that require defence by the phagocytes researched in the present study.
Individuals with atopic dermatitis frequently present recurrent infections from pyogenic bacteria or from intracellular microorganisms.
The mononuclear and polymorphonuclear neutrophilic phagocytes participate in the innate defence, acting quickly against different agents.1 These cells initially present chemotactic activity, migrating towards the chemotactic factors and then to the area where the immune response takes place. Following this, phagocytosis occurs, which consists in the ingestion and digestion of the pathogenic organisms, with subsequent elimination of their inactivated products.
Neutrophil impairment, in particular, reduces the defence against pyogenic bacteria such as Staphylococcus aureus, Streptococcus pyogenes, as well as Serratia marcenscens, Escherichia coli, Pseudomonas aeruginosa and Aspergillus. When mononuclear phagocyte disorders are present, infections involving intracellular organisms are more frequently observed, such as those caused by Mycobacterium tuberculosis, Candida sp, N. gypseum, T. tonsurans and viruses in general.1
Atopic dermatitis is part of an allergic diseases group, which are commonly observed by allergist.2 The prevalence of the allergic processes has been increasing considerably in the past decade, principally in the western countries, which have referred to this as “pandemic allergy”.3,4
Several studies have claimed that atopic dermatitis is the first manifestation of the atopic syndrome,3 also called the “Allergic Gait/March”, consisting of skin, digestive and respiratory airway manifestations. Almost all the cases of atopic dermatitis begin before the age of five, and are usually associated with a personal or a familiar history of atopy.2,5 Atopic dermatitis may evolve to food allergy, allergic rhinitis or bronchial asthma. Data suggest that the allergic triad of asthma, rhinitis and atopic dermatitis affects between 8 and 25% of the world population, being more predominant in urban zones.5 Atopic dermatitis interferes in one's quality of life and it is estimated that approximately 10–20% of children and 1–3% of adults are affected,6 reasons as to why this entity is always more important.
Atopic dermatitis presents itself as a chronic inflammatory skin process, with pruriginous eruptions and the tendency for recurrences. The diagnosis is mainly clinical, based upon major and minor criteria,7,8 responsive in the monitoring of Brazilian atopic dermatitis.8,9 Among the major criteria are pruritus, morphology and lesion distribution, the chronic and recurrent nature of the disease and prior family or personal history of atopies. Among the minor criteria are xerosis, ichthyosis, pilar keratosis, accentuated palmar creases, perioral and periauricular fissures, constant exofoliation of the scalp, white dermatographism, sudoresis, precocity of the disease, and the stigmas of the allergic individual presenting the infra-orbital Dennie-Morgan folds and Hertogue's sign.7,8 The disease has a prolonged course, with periods of improvement and exacerbations; during the acute phase, exudation occurs, and in the chronic phase the skin becomes dry with eczematous and lichenoid lesions. In all age groups the lesions tend to be symmetric.7
The physiopathology of atopic dermatitis is directly linked to immune disorders, with the predominance of type I hypersensitivity. There is an increase of Th2 cells, which synthesise IL-4, 5, 6, 9, 10, 13, and 19. Interleukins 4 and 5 allows the change of class to IgE and IL-6, with the subsequent afflux of eosinophils. The patient presents mastocytes with receptors having a high affinity for IgE (RFcεI). In the chronic phase, Th1 cells seem to play an important role because studies have shown positive cutaneous tests with late readings, suggesting cellular hypersensitivity.10
The high frequency of infections in individuals with atopic dermatitis suggests immune disorders, possibly involving the alterations of neutrophilic and mononuclear phagocytes.11–13 However, these alterations have not been defined. Forte et al. observed a deficiency in the activity of mononuclear phagocytes in five patients with atopic dermatitis.2 The recurrent infections by pyogenic bacteria or by intracellular organisms that occur in atopic dermatitis suggest that phagocytic activity disorders occur with greater frequency. Due to these findings, it is very useful to analyse the possibility of immune disorders in individuals with atopic dermatitis, since such comorbidities influence the disease evolution and require early diagnosis and specific treatment. In the literature, there are practically no studies about monocyte and neutrophilic activity in patients with atopic dermatitis.
The aim of this study is to evaluate phagocyte activity in laboratory conditions and of the chemotactic responses by neutrophilic and mononuclear phagocytes in the peripheral blood of patients with atopic dermatitis from moderate to severe.
Materials and methodsNineteen patients with moderate to severe atopic dermatitis between the ages of 2 and 20 years old were selected from the study group, who undergo ambulatory follow-up in the Allergy Sector. The laboratory evaluation was performed by the Immunology Section of the institution. The results were compared to those of healthy individuals within the same age group, without atopic or other disorders (control group). This transversal study was approved by the Research Ethics Committee.
The clinical study was made based on a protocol which was filled out by using the data collected from the patients’ charts and their ambulatory follow-up. The variables of the protocol included the following: age at time of disease diagnosis; presence of other allergic diseases (asthma, rhinitis, food allergies); prior familiar history of atopy; type of atopy; site and severity of the disease at the time of the initial diagnosis (SCORAD); types of dermatoses were excluded based upon the clinical aspects, laboratory exams, and, when necessary, skin biopsies.
In order to assess phagocyte activity, neutrophils were separated through spontaneous sedimentation at a temperature of 37°C, with the mononuclear cells separated by using the Ficoll-Hypaque gradient. To evaluate phagocyte ingestion, 2×106cells/mL were counted and three assays performed in Leighton tubes. In the first tube, the phagocytes were incubated with 108 particles of zymosan (Zy) per mL; in the second, the same concentration of phagocytes and Zy were incubated with 200μL of homologous serum pool (HS); and in the third, phagocytes, Zy and 200μL of autologous serum (AS). After 2h of incubation with CO2 at 5% at a temperature of 37°C, the number of phagocytes presenting three or more phagocytic vacuoles was counted within a fixed number of 200 phagocytes.14–18
For the assessment of chemotactic activity, analogous assays were used: control, incubated phagocytes with bacterial lipopolysaccharide (LPS) and HS and phagocytes, LPS and AS. The results were assessed according to the distance of cellular migration, measured in micrometers.14–18
The data obtained during material collection were also placed in tables in a specific protocol using the Microsoft Excel software (2002) for individualised analysis. A descriptive analysis of these variables was performed with the qualitative analysis presented in terms of absolute frequencies (n) and relative frequencies (%). The statistical analysis used was “t student” and was considered significant for p≤0.005. In relation to the quantitative variables, the measures and the respective standard deviations were calculated, as well as the confidence intervals of 95% (IC 95%).
ResultsNineteen individuals with atopic dermatitis were studied, being 11 female and 8 male. The patients’ average age was of 6.47 years (±4.56 years).
It was observed that the mean age at the time of diagnosis of atopic dermatitis was of 2.3 years (±3 years). Among the 19 patients in the study, 84.21% presented a personal history of atopy, with asthma in 57.89% and allergic rhinitis in 63.16%. With regard to familiar history, 63.16% described atopy in the family.
The clinical aspects at the time of diagnosis evidenced that the main signs and symptoms presented were: cutaneous xerosis (84.21%), pruritus (78.95%), eczematous lesions (68.42%) and lichenoid lesions (26.32%). As to the site of the lesions, the main regions affected were on the flexor regions (47.37%), facial area (36.84%), scalp (15.79%), the extensor regions (10.53%), and on the hands (5.26%). At the moment of diagnosis 26.32% of the patients presented lesions throughout their bodies.
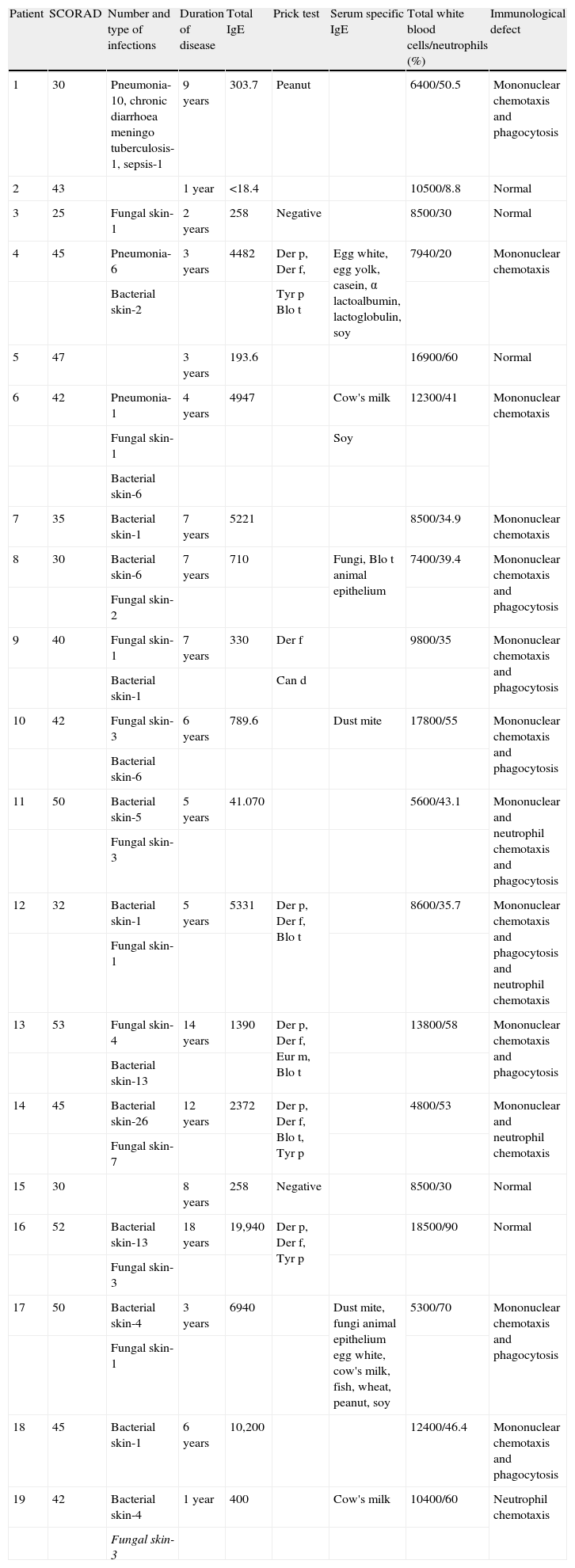
Table 1 shows the patients’ characteristics including SCORAD, the number and type of infections, duration of disease, total and specific IgE and/or prick test, blood cell count in particular number of white cell and neutrophils. It was shown that among the 19 patients studied we found that seven (36.8%) presented deficiency of mononuclear chemotaxis and phagocytes; three (15.7%) deficiency of mononuclear chemotaxis; one (5.26%) deficiency of neutrophils chemotaxis; one (5.26%) deficiency of both mononuclear and neutrophils chemotaxis and phagocytes; one (5.26%) deficiency of mononuclear chemotaxis and phagocytes and neutrophils chemotaxis; and one (5.26%) deficiency of mononuclear and neutrophils chemotaxis, whilst five (26.3%) did not present any deficiency.
Patients’ characteristics: number and type of infections, duration of disease, total IgE, prick test, serum specific IgE, total white blood cells and neutrophils and immunological defect
| Patient | SCORAD | Number and type of infections | Duration of disease | Total IgE | Prick test | Serum specific IgE | Total white blood cells/neutrophils (%) | Immunological defect |
| 1 | 30 | Pneumonia-10, chronic diarrhoea meningo tuberculosis-1, sepsis-1 | 9 years | 303.7 | Peanut | 6400/50.5 | Mononuclear chemotaxis and phagocytosis | |
| 2 | 43 | 1 year | <18.4 | 10500/8.8 | Normal | |||
| 3 | 25 | Fungal skin-1 | 2 years | 258 | Negative | 8500/30 | Normal | |
| 4 | 45 | Pneumonia-6 | 3 years | 4482 | Der p, Der f, | Egg white, egg yolk, casein, α lactoalbumin, lactoglobulin, soy | 7940/20 | Mononuclear chemotaxis |
| Bacterial skin-2 | Tyr p Blo t | |||||||
| 5 | 47 | 3 years | 193.6 | 16900/60 | Normal | |||
| 6 | 42 | Pneumonia-1 | 4 years | 4947 | Cow's milk | 12300/41 | Mononuclear chemotaxis | |
| Fungal skin-1 | Soy | |||||||
| Bacterial skin-6 | ||||||||
| 7 | 35 | Bacterial skin-1 | 7 years | 5221 | 8500/34.9 | Mononuclear chemotaxis | ||
| 8 | 30 | Bacterial skin-6 | 7 years | 710 | Fungi, Blo t animal epithelium | 7400/39.4 | Mononuclear chemotaxis and phagocytosis | |
| Fungal skin-2 | ||||||||
| 9 | 40 | Fungal skin-1 | 7 years | 330 | Der f | 9800/35 | Mononuclear chemotaxis and phagocytosis | |
| Bacterial skin-1 | Can d | |||||||
| 10 | 42 | Fungal skin-3 | 6 years | 789.6 | Dust mite | 17800/55 | Mononuclear chemotaxis and phagocytosis | |
| Bacterial skin-6 | ||||||||
| 11 | 50 | Bacterial skin-5 | 5 years | 41.070 | 5600/43.1 | Mononuclear and neutrophil chemotaxis and phagocytosis | ||
| Fungal skin-3 | ||||||||
| 12 | 32 | Bacterial skin-1 | 5 years | 5331 | Der p, Der f, Blo t | 8600/35.7 | Mononuclear chemotaxis and phagocytosis and neutrophil chemotaxis | |
| Fungal skin-1 | ||||||||
| 13 | 53 | Fungal skin-4 | 14 years | 1390 | Der p, Der f, Eur m, Blo t | 13800/58 | Mononuclear chemotaxis and phagocytosis | |
| Bacterial skin-13 | ||||||||
| 14 | 45 | Bacterial skin-26 | 12 years | 2372 | Der p, Der f, Blo t, Tyr p | 4800/53 | Mononuclear and neutrophil chemotaxis | |
| Fungal skin-7 | ||||||||
| 15 | 30 | 8 years | 258 | Negative | 8500/30 | Normal | ||
| 16 | 52 | Bacterial skin-13 | 18 years | 19,940 | Der p, Der f, Tyr p | 18500/90 | Normal | |
| Fungal skin-3 | ||||||||
| 17 | 50 | Bacterial skin-4 | 3 years | 6940 | Dust mite, fungi animal epithelium egg white, cow's milk, fish, wheat, peanut, soy | 5300/70 | Mononuclear chemotaxis and phagocytosis | |
| Fungal skin-1 | ||||||||
| 18 | 45 | Bacterial skin-1 | 6 years | 10,200 | 12400/46.4 | Mononuclear chemotaxis and phagocytosis | ||
| 19 | 42 | Bacterial skin-4 | 1 year | 400 | Cow's milk | 10400/60 | Neutrophil chemotaxis | |
| Fungal skin-3 |
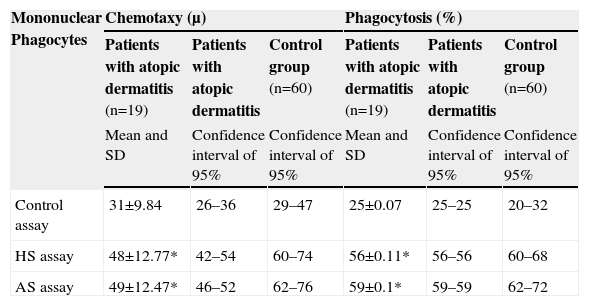
Table 2 shows the results observed in relation to the chemotactic and phagocytic activity by mononuclear phagocytes, the confidence interval of 95% of the measures found, the control group values, and the indication of a statistically significant difference. The results observed in relation to the chemotactic and phagocytic activity by neutrophilic polymorphonuclear cells are shown in Tables 3 and 4, separated according to age group—since distinct values were observed for the different age groups in this evaluation.
Distribution of the values of the means and standard deviations of the patients with atopic dermatitis and of the healthy individuals in relation to the chemotaxy by mononuclear phagocytes (migration distance in μ) and phagocytosis by mononuclear phagocytes (percentage of mononuclear cells that presented three or more phagocytic vacuoles within a total of 200 mononuclear cells)
| Mononuclear Phagocytes | Chemotaxy (μ) | Phagocytosis (%) | ||||
| Patients with atopic dermatitis (n=19) | Patients with atopic dermatitis | Control group (n=60) | Patients with atopic dermatitis (n=19) | Patients with atopic dermatitis | Control group (n=60) | |
| Mean and SD | Confidence interval of 95% | Confidence interval of 95% | Mean and SD | Confidence interval of 95% | Confidence interval of 95% | |
| Control assay | 31±9.84 | 26–36 | 29–47 | 25±0.07 | 25–25 | 20–32 |
| HS assay | 48±12.77* | 42–54 | 60–74 | 56±0.11* | 56–56 | 60–68 |
| AS assay | 49±12.47* | 46–52 | 62–76 | 59±0.1* | 59–59 | 62–72 |
HS=Homologous serum; AS=Autologous serum; SD=Standard deviation.
*Statistical analysis t student considered significant p≤0.005.
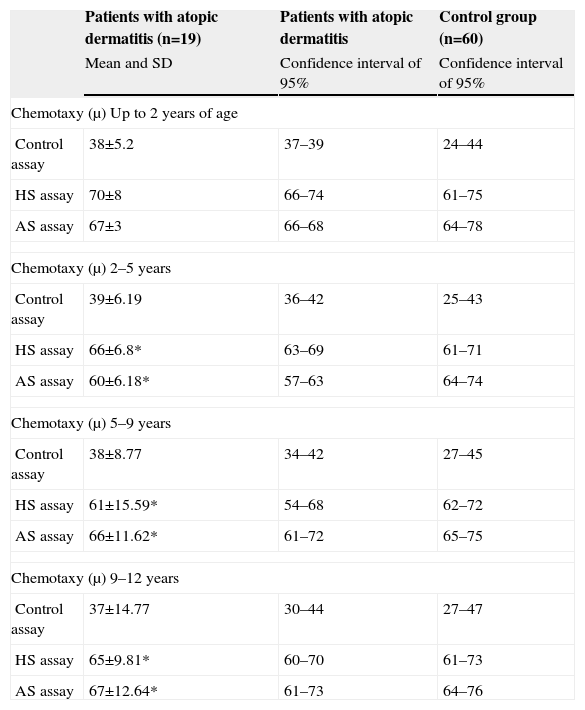
Distribution of the mean values and standard deviations of the patients with atopic dermatitis and the healthy individuals in relation to the chemotaxy by neutrophils (migration distance in μ)
| Patients with atopic dermatitis (n=19) | Patients with atopic dermatitis | Control group (n=60) | |
| Mean and SD | Confidence interval of 95% | Confidence interval of 95% | |
| Chemotaxy (μ) Up to 2 years of age | |||
| Control assay | 38±5.2 | 37–39 | 24–44 |
| HS assay | 70±8 | 66–74 | 61–75 |
| AS assay | 67±3 | 66–68 | 64–78 |
| Chemotaxy (μ) 2–5 years | |||
| Control assay | 39±6.19 | 36–42 | 25–43 |
| HS assay | 66±6.8* | 63–69 | 61–71 |
| AS assay | 60±6.18* | 57–63 | 64–74 |
| Chemotaxy (μ) 5–9 years | |||
| Control assay | 38±8.77 | 34–42 | 27–45 |
| HS assay | 61±15.59* | 54–68 | 62–72 |
| AS assay | 66±11.62* | 61–72 | 65–75 |
| Chemotaxy (μ) 9–12 years | |||
| Control assay | 37±14.77 | 30–44 | 27–47 |
| HS assay | 65±9.81* | 60–70 | 61–73 |
| AS assay | 67±12.64* | 61–73 | 64–76 |
HS=Homologous serum; AS=Autologous serum; SD=Standard deviation.
*Statistical analysis t student considered significant p≤0.005.
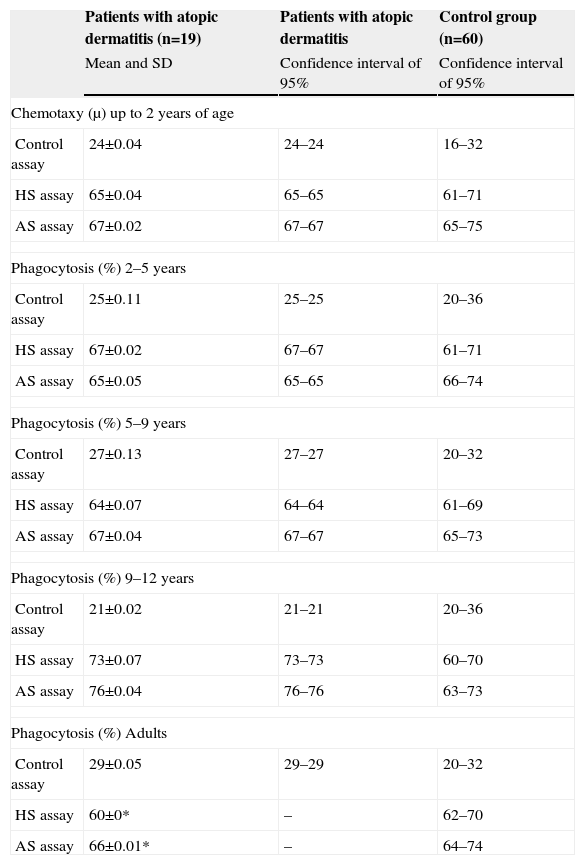
Distribution of the mean values and standard deviations of the patients with atopic dermatitis and healthy individuals in relation to phagocytosis by neutrophils (percentage of neutrophils that presented three or more phagocytic vacuoles within a total of 200 neutrophilic cells)
| Patients with atopic dermatitis (n=19) | Patients with atopic dermatitis | Control group (n=60) | |
| Mean and SD | Confidence interval of 95% | Confidence interval of 95% | |
| Chemotaxy (μ) up to 2 years of age | |||
| Control assay | 24±0.04 | 24–24 | 16–32 |
| HS assay | 65±0.04 | 65–65 | 61–71 |
| AS assay | 67±0.02 | 67–67 | 65–75 |
| Phagocytosis (%) 2–5 years | |||
| Control assay | 25±0.11 | 25–25 | 20–36 |
| HS assay | 67±0.02 | 67–67 | 61–71 |
| AS assay | 65±0.05 | 65–65 | 66–74 |
| Phagocytosis (%) 5–9 years | |||
| Control assay | 27±0.13 | 27–27 | 20–32 |
| HS assay | 64±0.07 | 64–64 | 61–69 |
| AS assay | 67±0.04 | 67–67 | 65–73 |
| Phagocytosis (%) 9–12 years | |||
| Control assay | 21±0.02 | 21–21 | 20–36 |
| HS assay | 73±0.07 | 73–73 | 60–70 |
| AS assay | 76±0.04 | 76–76 | 63–73 |
| Phagocytosis (%) Adults | |||
| Control assay | 29±0.05 | 29–29 | 20–32 |
| HS assay | 60±0* | – | 62–70 |
| AS assay | 66±0.01* | – | 64–74 |
HS=Homologous serum; AS=Autologous serum; SD=Standard deviation.
*Statistical analysis t student considered significant p≤0.005.
The comparison of the values of chemotaxy by mononuclear phagocytes of the study group to the control group demonstrated a significant reduction in all age groups with atopic dermatitis. The chemotaxy by polymorphonuclear neutrophils was significantly reduced in atopic dermatitis patients in the age groups among 2–5, 5–9 age range and over 12 when compared to the control group. The first assay (control), performed in order to evaluate chemotactic activity, verified the spontaneous migration of the cells and was used for the analysis of cell viability. In every case, there was a reduction in the phagocytic activity. In the second (cells incubated with Zy and a pool of normal human serum), and in the third (cells, Zy, and serum from the respective patient). The fact that both assays presented a reduction in the chemotactic activity, in addition to parallel studies demonstrating the C3 complement component of normal serum, excludes the possibility that the reduction in chemotactic activity is a problem related to the serum, homologous or autologous.
It was demonstrated that there was a reduction in the phagocytic activity by mononuclear phagocytes in patients with atopic dermatitis in all age groups studied. In the case of neutrophils, the same deficiency was observed only in patients with atopic dermatitis over 12 years of age. The first assay (control) was used to verify cell viability. The reductions observed for the phagocytic activity in the second and third assays evidenced an intrinsic problem of the cells. The phagocytosis of zymosan particles used in our methodology evaluates the phase of the ingestion by neutrophils and mononuclear cells, since both cells present receptors that permit opsonisation. The incubation of phagocytes with serum allows the complement to be activated through the zymosan used, resulting in the activation of the C3b and C5b components, which bind to these particles, opsonising them and allowing the ingestion of the same by phagocytes with receptors for C3b and C5b, such as monocytes and neutrophils.
The techniques applied for chemotaxis analysis and the ingestion phase of phagocytosis in the present study had already been used in prior studies, demonstrating clinical correlations in different situations.2,6,14–18
It was shown that among the patients studied those who presented the smallest number of infections were those who did not show phagocyte deficiency. Among them, one had several skin infections, probably because this patient has had a longer follow-up time, besides been a patient who lives in bad living conditions. The patients studied with atopic dermatitis who had deficiency of chemotaxis and/or phagocytises activity presented recurrent bacterial and fungi infections, and one of then presented severe systemic infections.
It is known that a great number of patients with atopic dermatitis present recurrent infections by Staphyloccocus aureus, Streptoccocus pyogenes and fungi. It is known that the toxins produced by Staphyloccocus aureus may act as superantigens in atopic dermatitis, which may promote the activation of T lymphocytes regardless of the association of the epitope with the HLA,5 resulting in exacerbated lymphocytic proliferation with consequent tissue damage. Little is known about the immune disorders in atopic dermatitis. However, it has been established that the skin of such patients is frequently colonised by Streptoccocus and Staphyloccocus, demonstrating that these bacteria are not removed by either aerial or liquid flow. If there is a disorder in the patient's immune system with atopic dermatitis, especially one affecting neutrophilic and mononuclear phagocytes, the patients may develop an infectious site from these pathogens with greater facility.
Our results, which demonstrated a reduction in the activity of mononuclear and neutrophilic phagocytes, are coherent with the hypotheses presented in the literature, in which patients with atopic dermatitis present these immune disorders.19–21 Our results are in accordance with the recurrent infectious processes presented by these patients, such as pyogenic bacteria and fungi, microorganisms that require defence by the phagocytes studied.
We conclude that there is a reduction in chemotactic response and the phagocytic activity by neutrophilic and/or mononuclear phagocytes in the majority of patients studied with atopic dermatitis from moderate to severe.
We believe that investigation regarding immune disorders in patients with atopic dermatitis is very important since it may contribute to an adequate treatment of this disease and improve these patients’ quality of life.