INTRODUCTION
During the last years, the evaluation of the Quality of Life (QL) has been considered as an important subject in clinical investigation. It is consensus that the study of QL should be incorporated in clinical studies as an important tool, mainly as regards mortality and survival rates. It could be a topic of interest in the interface between studies in Medicine and Psychology (1).
In daily practice, it can be used for measuring the contribution of the clinical management of reduction in chronic disease impact on the patient's daily life. QL evaluation has been shown useful, also for monitoring the effectiveness of a specific treatment in disease management. QL also allows the physician familiar with patient evaluation through parameters, such as physical disposition, vigor and ability to perform routine activities, in order to approach the patient's psychosocial universe, thus being able to see it in an integral manner.
The increased use of instruments to evaluate QL, has created an "illusion of simplicity" that led researchers to consider it an easy and simple measurement (2). However, difficulties begin starting with its concept. Mostly, the definition is generic, therefore being considered from a general point of view. In the wide concept, QL is a group of physical and psychological characteristics, experimented in the social context and in agreement with the individual lifestyle (3, 4). In a restricted context of health, QL is based on more objective and measurable data, with respect to the limitation and discomfort produced by the disease itself and/or its treatment (3).
In this new research field, the binomial, chronic disease and QL, is indeed more explored by the specialists. It is known that psychological reactions to chronic diseases may be felt at different levels, varying from person to person. A disease with a prolonged course deprives the individual of a lot of personal pleasent sources, as it interferes in the self-respect, in the control of the own body and in interpersonal relationships (5). The repercussions of a chronic disease in children and adolescents also affect their family as a whole, and the consequent long-term problems may further damage the QL of the whole group.
Allergic diseases are the more prevalent chronic diseases in childhood. In perennial allergic rhinitis (PAR) patients, the persistence of symptoms causes limitations in the patient's daily activities, not only due to the discomfort originated by the nasal symptoms, as well as due to the associated symptoms, such as: migraine, indisposition, reduction in the capacity of concentration, etc... The limitations to social conviviality, sometimes, erroneously interpreted as indifference, apathy, inattention, mainly of the school age, rebound negatively on the emotional welfare of these patients, with an important reflex in QL (6-8).
The studies relating PAR and QL evaluation are still few, and they use written questionnaires (WQ) developed and published in the English language (9, 10). The objective of this study was to adapt to the Portuguese language (Brazilian culture), the RQLQ (Rhinoconjunctivitis Quality of Life Questionnaire) WQ (11), destined for QL evaluation in teenagers with allergic rhinitis.
PATIENTS AND METHODS
Selection
In this phase, the previously published 75 items (11) were translated into Portuguese (Brazilian culture). Some alterations were made in order to adapt expressions or words, and to suppress some of them considered to be repetitive. Ocular symptoms were reduced, considering only the most frequent in PAR patients. Fifty-seven PAR adolescents, regularly enrolled and followed-up, at least for one year, in the Division of Allergy, Clinical Immunology and Rheumatology of the Department of Pediatrics of the Universidade Federal de São Paulo-Escola Paulista de Medicina (UNIFESP-EPM), were interviewed. They were requested to mark the daily suffered situations, always pointing out their relationship with the rhinitis picture. They were requested also to grade them using a score varying between 1 (not very disturbed) and 4 (extremely disturbed). As standardized by Juniper et al. the Global Importance (GI) of each question was calculated (11). Those who reached values equal to or above 1,0 composed the final WQ respecting the basic norms of elaboration of instruments to evaluate QL (9, 11, 12).
This study was approved by the Ethics Committee of UNIFESP-EPM and all patients participated after signed informed consent by their parents or guardians.
Elaboration of the Questionnaire
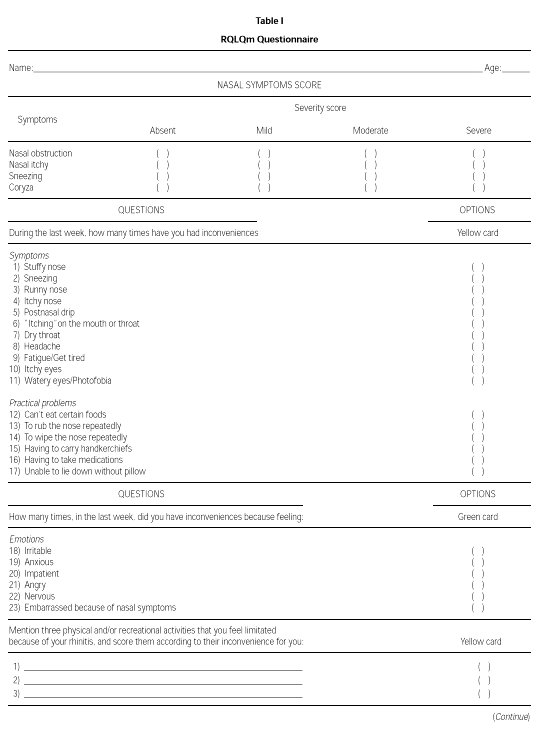
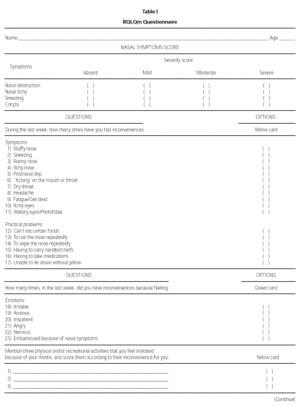
The modified WQ (RQLQm) was composed of 26 items divided into four domains: Symptoms, Practical Problems, Emotions and Activities (table I). The evaluation of each symptom was done using a seven feel point scale, in which 1 means "I don't feel discomfort" and 7 means "disturbed me extremely" (yellow card), or 1 means "I don't feel discomfort" and 7 means "disturbed me all the time" (green card).
The RQLQm's reproducibility was evaluated applying the WQ to a group of PAR adolescents, aged from 11 to 17 years, on two different occasions, two weeks apart. These patients were sensitive (skin prick test, mean wheal diameter greater than 3 mm) to one or more aeroallergens: D. pteronyssinus, D. farinae, B. tropicalis, A. fumigatus, A. alternata, dog or cat epithelia. Between the evaluations, the patients were mantained without any specific medication or immunotherapy, except for environmental control.
To validate the RQLQm WQ, 42 PAR patients (19 males) were interviewed and were randomly selected to receive one of the following treatment regimens: A - loratadine (oral, 10 mg/day) or B: beclomethasone dipropionate (nasal spray, 400 mcg/day). The RQLQm WQ was applied by the same author (MGN) at admission, at 2 and at 4 weeks after the beginning of the treatment. At all these evaluations, patients were requested to give a score to their nasal symptoms (itch, obstruction, coryza and sneeze): absent (1 point), mild (2 points), moderate (3 points) and severe (4 points). At the second and third evaluations, patients were asked to quantify their improvement using a 15 point scale where 7 corresponds to extreme worsening and 7 to extreme improvement.
The statistical analysis were performed using non-parametric tests: Wilcoxon's test, Mann Whitney's test, Friedman's analysis of variance by ranks complemented by Dunn's test, Spearman's coefficient correlation and Kruskal-Wallis' analysis of variance by ranks (13, 14). The level of significance to reject the null hypotesis was 5 %.
RESULTS
Validation
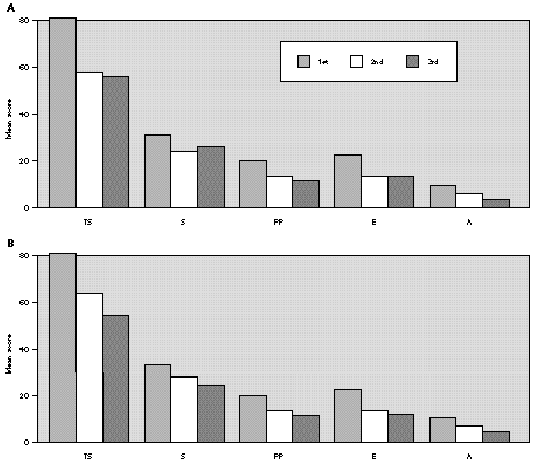
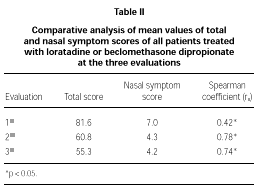
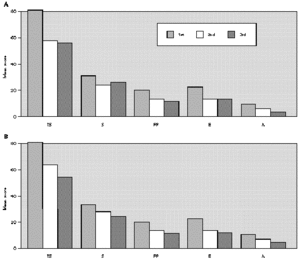
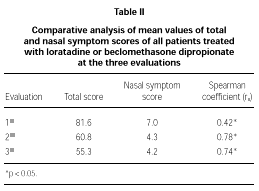
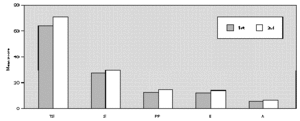
The comparative study between the points obtained at the three evaluations showed statistically significant differences with progressive fall in total and domain scores along the evaluations, for both A and B groups (fig. 1A and B). There were no significant differences between total score for Group A and B, at the three evaluations (fig. 1A and B). There was a positive correlation between the total score and the grade attributed to the severity of the nasal symptoms (table II).
Figure 1.--Mean score values: Total (TS) and Domains: Symptoms (S), Practical Problems (PP), Emotions (E), Activities (A) obtained at three evaluations of patients treated with loratadine (A) or beclomethadone dipropionate (B). Friedman's test: group a: TS, S, PP, E and A 1st > 2nd, 3rd; group b: TS, A 1st > 2nd, 3rd; S, E, PP 1st > 3rd; Mann-Whitney test: group a ×group b; TS: 1st, 2nd, 3rd a = b.
Reproducibility
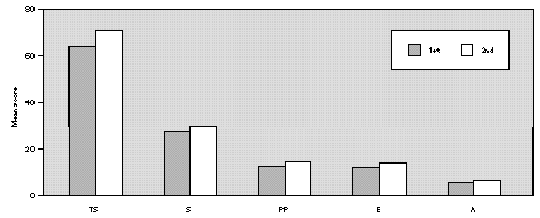
The analysis of the total and domains scores, obtained at two different occasions, before and two weeks after start of the treament, did not show significant differences (fig. 2).
Figure 2.--Mean score values: Total and Domains: Symptoms (S), Practical Problems (PP), Emotions (E) and Activities (A) obtained at two evaluations two weeks apart, in the same patients. Wilcoxon test: All scores 1st = 2nd (p < 0.05).
Responsiveness
To evaluate the responsiveness, the patients were evaluatetd by the percentual total score variations of the QLRQm WQ observed at the first and second evaluations. They were distributed in three groups according to the grade attributed to the clinical evolution: Group 1: grade between 1 and + 1; Group 2: grade between 3 and 2 or + 2 and + 3; Group 3: grade between 7 and 4 or + 4 and + 7. There were no significant differences between the three groups.
DISCUSSION
The expression Health-Related Quality of Life, nowadays, has been frequently mentioned in the literature as a useful parameter to evaluate the impact of a certain disease and/or its treatment on the patients day-by-day (15, 16). The acquisition of a standardized method of interpretation (grades or visual scales) allowed an objective quantification of these data, previously obtained as part of the anamnesis, and their comparison with other clinical parameters and/or laboratory data (16).
In this field, the WQ has been the mainly utilized tool. The questionnaires have a good acceptance, low cost and do not request special equipment for their utilization. The first WQ developed with the purpose of evaluating QL was generic (17). Initially, the specific WQ was used to evaluate patients with disabling diseases and/or bad prognosis or still those with diseases that need continuous treatment (osteoarticular diseases, oncohematologic diseases, diabetes, hypertension or chronic renal diseases) (6, 18).
The study of QL in allergic diseases began in the last decade. Initially they only focused on asthma (19, 20). With the publication of a WQ for evaluation of the asthmatic patient's QL (21), there was an increasing interest and this instrument was translated, adapted and validated by several researchers in different languages (22-24).
Regarding allergic rhinitis, as previously mentioned, there were few studies and they used the tools developed by Juniper et al (9, 11). One of them was designed to evaluate adolescent QL and we used it as reference for our study. As recommended, in order to use an instrument developed in another language, it must be translated and applied as a whole or, alternatively, submitted to a selection of the most relevant propositions contained in the original work, with an active participation of the study population.
To make the RQLQ more adjusted to our population, instead of its translation into Portuguese, it was submitted to a selection of the most relevant items. So, in the first phase of the study 57 PAR adolescents were interviewed and asked to point out those original propositions that cause discomfort to them. Afterwards, as described by Juniper et al (11), the global importance of each subject was calculated and the modified RQLQ version was obtained. The RQLQm was composed of 26 items divided into four domains as the original one. The RQLQm's content was similar to other WQ used by other investigators.
Nasal symptoms were the predominantly referred to however others, such as: migraine, indisposition, and thirst were also frequently mentioned. Many adolescents mentioned to feel rage, being anxious, nervous, uncomfortable to use medication continually, and embarrassed because their symptoms. Reduction in the capacity of concentration and insomnia were less important in our sample (25).
Regarding physical activities and leisure, there were less limitations in our study than those observed by the authors of original RQLQ (11). The cultural differences and the great variety of personal preferences may be responsible for the observed differences.
The usefulness of a WQ must be evaluated by the study of its validation, reproducibility and responsiveness. Acording to Guyatt (25), if during the elaboration or adaptation of a tool for QL evaluation, a previous selection of items is performed by the study population, the content validity is already characterized. However, to study the constructive validity, the comparison of the WQ results obtained by WQ with clinical and/or laboratory parameters utilized for the disease diagnosis was necessary (26). In the absence of this gold standard, the constructive validity must be studied evaluating the effect of a recognized effective treatment on the score obtained by WQ (16, 17, 27).
In our study, the constructive validity of RQLQm was double-checked. Initially, we evaluated the effect of two treatment regimens usually used in PAR patients. It was supposed that clinical improvement, secondary to the established treatment, follows a fall in the degree of QL damage, pointed out by those patients. We also studied the correlation between the severity of nasal symptoms and total RQLQm score along the treatment, as observed by others (9, 22, 27-30).
The RQLQm's reproducibility was evaluated applying it, on two occasions 2 weeks apart, to a group of patients without treatment. There were no significant differences between them considering the total or domain scores (fig. 2). These data show that the RQLQm is reproducible.
In opposition to others, we did not demonstrate the responsiveness of RQLQm. There were no differences between the clinical evolution (patient's subjective evaluation) and the differences between RQLQm WQ scores at the first and second evaluations. Although topical glucocorticosteroids are the most effective treatment for PAR we did not observe statistical significant differences between the two treatment regimens (31, 32).
In conclusion, RQLQm is an useful tool for the evaluation of PAR patients, mainly those participating in longitudinal studies. It is reproducible, valid, and capable to detect possible clinical improvement of treatment. The RQLQm total and domain scores correlate positively with clinical improvement and with nasal symptoms scores, along the treatment (longitudinal constructive validity). Subjetive symptom severity evaluated by the patients themselves may be considered useful to asses evolution of the disease, when we do not have objective laboratory and clinical measurements. We could not demonstrate RQLQm responsiviness, comparing the evolution of the disease and total score. The RQLQm should be submitted to other investigations to consolidate its properties.