INTRODUCTION
A subpopulation of patients with chronic urticaria experience flares of hives after taking several, chemically unrelated nonsteroidal anti-inflammatory drugs (NSAID) (1-3). The prevalence of NSAID-reactivity among patients with chronic urticaria ranges between 21 % to 30 % (1, 4-6); interestingly, in these subjects susceptibility to NSAID varies with degree of urticaria activity (3). The causes for such NSAID reactivity are still elusive. Recent studies have shed new light on the pathogenesis of chronic idiopathic urticaria showing that many patients are characterized by autoreactivity on autologous serum test and that histamine release may be induced by autoantibodies directed against the a subunit of the high affinity IgE receptor (FceRI) or against IgE (7-12). NSAID intolerance in patients with chronic idiopathic urticaria has not been investigated in the light of these findings yet. The present study aimed to evaluate the susceptibility to NSAID in different subgroups of patients with chronic urticaria in view of the histamine-releasing activity of their sera.
PATIENTS AND METHODS
Patients
117 adults (M/F 41/76; mean age 37 years) with chronic urticaria, defined as recurrence of hives with or without angioedema for more than 6 weeks, were studied. A careful interview was carried out in order to detect patients who experienced exacerbations after taking NSAID.
Skin tests
Patients underwent intradermal test with 0.05 ml of sterile, fresh autologous serum and with saline as negative control. A skin prick test with histamine 10 mg/ml was used as positive control. Readings were taken at 15 and 40 min. Only an unequivocal wheal formation in response to serum injection was considered as a positive test. All intradermal tests were performed at least 5 days after the ingestion of the last antihistamine tablet (cetirizine 10 mg, fexofenadine 180 mg, or loratadine 10 mg in all cases) and during a phase of moderate to strong clinical activity of the disease (9). After the skin tests, patients' sera were stored at 20 °C for subsequent histamine release assays.
Basophil histamine release assay (HRA)
Leukocyte suspensions from normal donors were prepared by dextran sedimentation of peripheral venous blood, anticoagulated with 0.01 M EDTA, and mixed with 6 % dextran in saline solution (Solplex 70, Sifra, Verona, Italy) and 30 mM dextrose (Sigma Chemical, St Louis, MO, USA). The cells were allowed to settle for 60-90 min at room temperature, according to Lichtenstein et al (13). The leukocyte-rich plasma was aspirated and centrifuged at 300 g for 15 min at 4 °C, and the cell button was washed twice in Hepes-buffered saline solution, pH 7.4, containing (mM): NaCl 140, dextrose 5.5, KCl 5, Hepes 5. Leukocytes (with about 7 * 104 basophils) were then re-suspended in 100 ml volumes of Hepes-buffered saline solution with 1.8 mM CaCl2 and 0.5 mM MgCl2, and incubated with sera at the dilution of 1:2, making a final volume of 200 ml. After incubation for 40 min at 37 °C, the reaction was stopped by addition of 800 ml of ice-cold buffer solution and centrifugation at 1,000 g for 10 min at 4 °C. After centrifugation, the supernatants were aspirated, mixed with an equal volume of 6 % HClO4 and centrifuged at 1,000 g for 10 min at 4 °C. Histamine concentration in the supernatants was measured by an automated fluorometric method, according to Ruff et al (14). Spontaneous histamine release was evaluated by measuring histamine concentration in the supernatant of unstimulated cells, incubated for 40 min at 37 °C. Total histamine content was obtained by adding 100 ml of 6 % HClO4 to 100 ml of cell suspension. Net histamine release was calculated as percentage of total histamine content, after subtraction of spontaneous release. A 5 % release cut-off value was used. Sera were tested with basophils from three normal donors, showing 30 % net histamine release on challenge with an optimal dose of anti-IgE (10 ml/ml; Sigma Chemicals, St Louis, MO, USA).
RESULTS
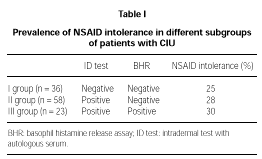
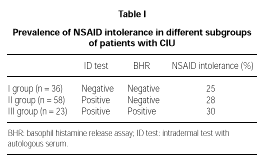
32/117 (27 %) patients reported NSAID intolerance (i.e. exacerbation of their disease after taking NSAID). On the basis of intradermal tests and HRA results, CIU patients were divided into 3 subgroups (table I):
1. Negative both on skin and serological tests (n = 36). 9 (25 %) of these patients did not tolerate NSAID.
2. Positive on intradermal test but negative on HRA (n = 58). Such condition has been associated with a circulating mast cell-specific soluble factor as a possible cause of histamine release (10). 16 (28 %) of these subjects had a history of NSAID intolerance.
3. Positive on both intradermal test and HRA (n = 23). This condition has been associated with circulating autoantibodies to FcεRI or IgE (8-11). 7 (30 %) of these subjects has a history of NSAID intolerance.
The prevalence of NSAID intolerance was almost identical in the three subgroups.
DISCUSSION
The overall prevalence of intolerance to NSAID among our patients was very similar to that observed by others authors (20-30 %) (1, 4-6), which shows that our population was representative of patients with CIU. The prevalence of NSAID intolerance was nearly identical in three subgroups of urticaria patients distinguished on the basis of different in vivo and in vitro histamine releasing properties of their sera; this shows that in patients with CIU, intolerance to NSAID does not depend on the mechanism of histamine release (whether induced by autoantibodies to FceRI or IgE or by other hitherto not defined factors). Recently, an overexpression of LTC4 synthase, a key enzyme in cysteinyl leukotrienes production (16, 17), was observed in about 70 % patients with aspirin-induced asthma (AIA), and this has led to hypothesize that COX-inhibition may be responsible for NSAID-induced asthma attacks in AIA patients. Good results on both skin disorder and NSAID intolerance have been reported in some patients with CIU by the use of leukotriene receptor antagonists (18-20). Moreover, CIU patients show a chronic activation of the basophil/mast cell system (21, 22), and their sera may induce histamine release as well as de novo LTC4 production by basophils of normal subjects (23). Altogether, these observations suggest that LTC4 overexpression could underlie NSAID-induced exacerbations of chronic urticaria as well. A study aiming to investigate this aspect is still in progress; unfortunately, preliminary (unpublished) results do not seem to support this hypothesis. At present, the reasons why one fourth of patients with chronic urticaria experience flares of hives after taking chemically unrelated NSAID remain undefined.