Asthma is characterised by chronic airway inflammation, a complex cascade of events, mostly sustained by eosinophil recruitment and activation. Fractional exhaled nitric oxide (FeNO) is a surrogate marker of airway inflammation closely associated with bronchial eosinophilia. FeNO is used to define asthma phenotype, to assess eosinophilic inflammatory severity and to predict corticosteroid responsiveness.
ObjectiveThe aim of this study was to investigate whether FeNO may be associated with some clinical and functional factors in asthmatics evaluated in a real life setting.
MethodsGlobally 363 patients (150 males, mean age 46.3 years) with asthma were consecutively evaluated. The following parameters were assessed: history, including comorbidities, physical examination, body mass index (BMI), lung function, asthma control grade, asthma control test (ACT), and FeNO.
ResultsFeNO values were significantly higher in patients with poorly controlled asthma (p<0.01), asthma symptoms (p=0.015), wheezing (p<0.001), rhinitis diagnosis, (p=0.049) and rhinitis symptoms (p=0.019), but lower in patients with GERD (p=0.024) and pneumonia history (p=0.048). FeNO values increased in patients with the lowest corticosteroid dose (p=0.031). FeNO values>25ppb were associated with poorly controlled asthma (OR 3.71), asthma signs (OR 3.5) and symptoms (OR 1.79). A FeNO value cut-off of 29.9ppb was fairly predictive of (AUC 0.7) poorly controlled asthma.
ConclusionsFeNO assessment in clinical practice may be a useful tool for monitoring asthmatics as it is associated with several clinical factors, including asthma control.
Asthma is a chronic disorder, characterised by three main pathophysiological characteristics: airways inflammation, variable and reversible bronchial obstruction, and bronchial hyper-responsiveness (BHR) to different stimuli.1 In addition, upper airways inflammatory disorders, including allergic rhinitis (AR), are frequently associated with asthma.2 Hence, airways inflammation represents a common pathogenic pathway. In this regard, the accurate measurement and monitoring of airways inflammation should be a crucial topic in managing patients with asthma as inflammation induces bronchial obstruction.3 Unfortunately, a direct assessment of bronchial inflammation needs invasive methods (e.g. bronchial biopsy and/or lavage) or sputum analysis; both are complex and require adequate setting. However, it is actually possible to evaluate bronchial inflammation also by measuring a surrogate marker, such as the fractional concentration of exhaled nitric oxide (FeNO), simply during an office visit.4,5 There is evidence that FeNO levels correlate well with peripheral or bronchial eosinophilia,6,7 thus it can be considered a marker of eosinophilic asthmatic inflammation. Recently, the determinants of exhaled NO in atopic asthma have been defined.7 In particular, the role of inducible nitric oxide synthase isoform in the airway inflammatory pathway has been investigated and well documented.7–9 In addition, a previous study showed that endogenous airway acidification during asthma attack was able to induce increased FeNO levels10, which, in theory, might also influence disease control in asthmatic patients with gastro-oesophageal reflux disease (GERD).
Asthma control is the long-term goal of asthma management even though many patients have sub-optimal or poor control, despite the large diffusion of international guidelines and availability of effective medications.1,11 In asthmatics it might be appropriate to assess and to monitor airways inflammation by measuring non-invasive biomarkers in order to achieve asthma control. Studies investigating this issue are still conflicting,7,12,13 as most of these studies are conducted on small numbers of patients or in experimental settings. Therefore, the present study aimed to evaluate whether FeNO measurement may relate with clinical and functional parameters in a cohort of patients with asthma observed in a real life setting.
Materials and methodsPatientsThis cross-sectional study included 363 patients suffering from asthma. They were outpatients, who referred to an asthma or allergy clinic for a specialist visit, and were sequentially recruited. The visit included history, clinical examination, lung function testing, FeNO measurement, asthma control test (ACT) questionnaire, and asthma control assessment. The San Luigi Hospital Review Board approved the study procedure and written informed consent was obtained from each subject.
Study design and settingAsthma and AR diagnosis was performed according to International guidelines.1,2 Inclusion criterion was asthma diagnosis, based on a history of intermittent wheezing, breathlessness, cough, and chest tightness in combination with reversibility to bronchodilators and/or BHR to methacholine (MCH). Exclusion criteria were: history of lung disease other than asthma, coronary artery disease, congestive heart failure, or cor pulmonale, recent asthma exacerbation, presence of acute (in the previous four weeks) or chronic upper and/or lower respiratory infections. Patients discontinued use of long-acting bronchodilators for 12h before measurement of lung function. Enrolment was sequential.
Age, gender, presence of comorbidities, including allergy, AR, GERD diagnosed according to validated criteria, pneumonia, referred asthma and/or AR symptoms in the last month, inhaled corticosteroids dosages, presence of asthma signs, mainly wheezing, at physical examination, FVC, FEV1, FEV1/FVC, FEF25–75, FeNO, asthma control level, and asthma control test (ACT), were registered for all patients in the analysis.
Functional assessmentSpirometry was performed using a computer-assisted spirometer (Pulmolab 435-spiro 235, Morgan, England, – predictive values ECCS 1993), with optoelectronic whirl flow meter. This spirometer fulfils the ATS/ERS standards according to guidelines.14,15 It was performed as stated by the European Respiratory Society.15
Nitric oxide measurementFeNO was measured with a chemiluminescence analyser (Eco Medics CLD88 sp, Duernten, Switzerland) before spirometry; the detection limit of the apparatus was 1–5 parts per billion (ppb), as required by ATS guidelines, with a resolution of 1ppb. The analyser was calibrated daily, using a certified NO mixture. Exhaled NO was recorded with the single-breath method according to published guidelines.16 Subjects inhaled to total lung capacity from NO-free air and exhaled a single breath (without nose clip) through a mouthpiece at a mouth pressure of 45cm water and at an expiratory flow of 50ml/s. Mouth pressure was displayed on a computer screen in order for the patients to maintain a steady flow. The measurement was rejected if a stable flow was not maintained for at least 6s of exhalation. Nitric oxide was measured at the plateau and expressed in ppb. A FeNO value>25ppb was considered pathological according to validated guidelines.16
Asthma control levelAsthma control level was assessed according to the last GINA guidelines: patients were classified as having: controlled, partly controlled, or poorly controlled asthma.1
Asthma control testACT questionnaire consisted of five questions with five possible responses, exploring the patient's perception of his/her asthma control.17 The result could range between 0 and 25, where 25 was the optimal asthma control.
AllergyAllergy was considered if there was a documented positivity of allergen-specific IgE, assessed by skin prick test and/or measurement on serum, and demonstration of a cause/effect relationship between exposure to sensitising allergen and symptom occurrence.
RhinitisRhinitis was considered if there was a history of typical nasal symptoms, such itching, sneezing, watery rhinorrhoea, and nasal obstruction. If the symptom occurrence was consistent with the exposure to sensitising allergen the diagnosis of allergic rhinitis was made.
Statistical analysisDemographic and clinical variables were expressed as mean±standard deviation, median with interquartile range, and frequency with percentage.
Frequencies of categorical variables were compared between groups by use of the Chi Square test with odds ratio and 95% confidence intervals.
The distribution of continuous data was assessed by the Shapiro–Wilk test.
Because of non-normal distribution, differences in continuous variables across groups were tested using the Mann–Whitney U test or the Kruskal–Wallis test.
Any significant association between continuous variables was measured by Spearman's rank-order correlation coefficient.
A non-parametric receiver operating characteristic (ROC) curve was generated. For each ROC curve the area under curve (AUC) with the corresponding 95% CI was plotted and the best cut-off for FeNO values provided both the highest sensitivity and the highest specificity for predicting not controlled asthmatic patients.
All statistical tests were two-sided, and the significance level (alpha error) was set at 0.05.
Analyses were carried out using the SPSS software package (version 21.0; SPSS Inc., Chicago, IL, USA).
ResultsBaseline demographic and clinical characteristics are shown in Table 1; statistically significant results of any relationship between FeNO and other variables are reported in Table 2.
Baseline characteristics (N=363).
| Age (yrs) | 46.28±17.11 45.00 [33.00–60.00] |
| Males, n (%) | 150 (41.3) |
| BMI | 25.31±5.18 24.69 [22.00–28.03] |
| BMI (The International Classification of adults, WHO) | |
| <18.50 (underweight) | 13 (3.5) |
| 18.50–24.99 (normal range) | 167 (46) |
| 25.00–29.99 (overweight) | 112 (31) |
| ≥30.00 (obese) | 71 (19.5) |
| FVC % of predicted | 99.36±18.71 99.50 [88.00–110.00 |
| FVC | |
| ≤80% | 51 (14) |
| >80% | 312 (86) |
| FEV1% of predicted | 88.00±21.13 90.00 [77.53–102.00] |
| FEV1 | |
| ≤80% | 112 (31) |
| >80% | 251 (69) |
| FEV1/FVC | 0.75±0.13 0.77 [0.66–0.84] |
| FEV1/FVC | |
| ≤0.70 | 122 (33.6) |
| >0.70 | 241 (66.4) |
| FEF25–75% of predicted | 64.02±30.83 64.50 [40.00–87.00] |
| FEF25–75 | |
| ≤65% | 180 (49.5) |
| >65% | 183 (50.5) |
| FeNO | 30.73±24.85 22.00 [13.80–40.00] |
| FeNO | |
| ≤25 | 203 (55.9) |
| >25 | 160 (44.1) |
| Allergy, positive, n (%) | 295 (81.3) |
| Polysensitisation, positive, n (%) | 212/295 (71.9) |
| Rhinitis, diagnosis, n (%) | 328 (90.4) |
| Asthma signs, n (%) | 47 (12.9) |
| Asthma symptoms, n (%) | 242 (66.7) |
| Rhinitis symptoms, n (%) | 277 (76.3) |
| Asthma Control Test (ACT) | 20.08±4.39 21.00 [17.00–24.00] |
| Asthma control | |
| Poorly controlled | 41 (11.3) |
| Partly controlled | 175 (48.2) |
| Controlled | 147 (40.5) |
Values are expressed as mean±standard deviation, median (25th–75th), frequency (%).
Statistically significant associations between FeNO and clinical and medical history variables.
| FeNO≤25 | FeNO>25 | OR (95% CI) | p | |
|---|---|---|---|---|
| Asthma control | ||||
| Controlled | 89 (45.4) | 56 (35.2) | Ref. | 0.002* |
| Partly controlled | 95 (48.5) | 75 (47.2) | 1.26 [0.80–1.97] | |
| Poorly controlled | 12 (6.1) | 28 (17.6) | 3.71 [1.74–7.89] | |
| Wheezing | ||||
| No | 187 (93.0) | 126 (79.2) | Ref. | <0.001* |
| Yes | 14 (7.0) | 33 (20.8) | 3.50 [1.80–6.80] | |
| Asthma symptoms | ||||
| No | 79 (38.9) | 42 (26.3) | Ref. | 0.015* |
| Yes | 124 (61.1) | 118 (73.8) | 1.79 [1.14–2.81] | |
| Rhinitis diagnosis | ||||
| No | 25 (12.3) | 10 (6.3) | Ref. | 0.049* |
| Yes | 178 (87.7) | 150 (93.8) | 2.11 [0.98–4.53] | |
| Rhinitis symptoms | ||||
| No | 58 (28.6) | 28 (17.5) | Ref. | 0.019* |
| Yes | 145 (71.4) | 132 (82.5) | 1.89 [1.13–3.14] | |
| GERD | ||||
| No | 63 (69.2) | 60 (85.7) | 2.67 [1.19–5.96] | 0.024* |
| Yes | 28 (30.8) | 10 (14.3) | Ref. | |
| Pneumonia | ||||
| No | 72 (80.0) | 63 (91.3) | 2.63 [0.98–7.02] | 0.048* |
| Yes | 18 (20.0) | 6 (8.7) | Ref. | |
| ICS dose (μg) | 400.00 [200.00–600.00] | 250.00 [200.00–425.00] | 0.031* | |
Values are expressed as frequency (%) and odds ratio (OR) with 95% confidence interval (95% CI).
Statistically significant differences in FeNO were evidenced for the following clinical variables: “asthma control”, “wheezing”, “asthma symptoms”, “rhinitis diagnosis”, “rhinitis symptoms”, “GERD”, and “pneumonia”.
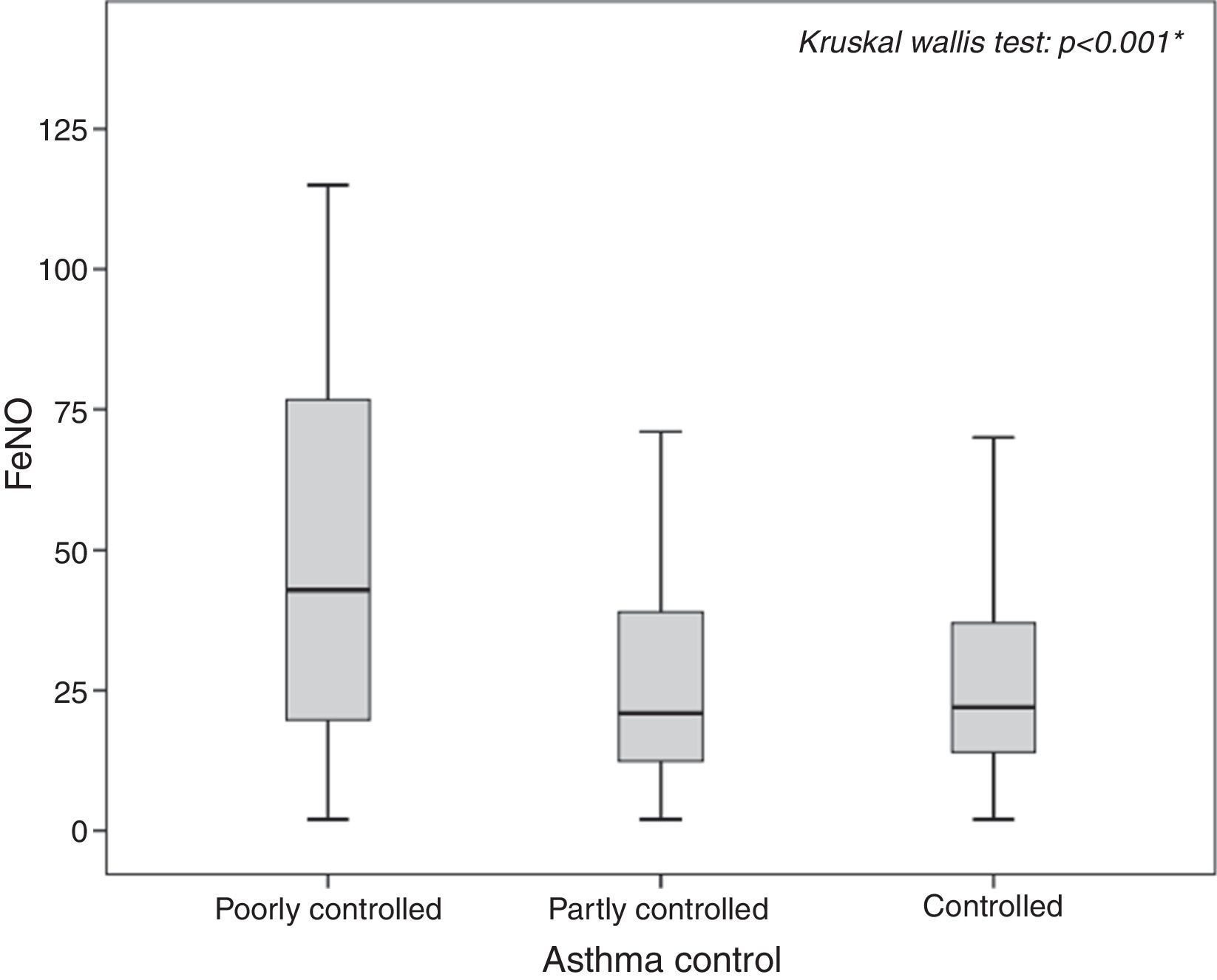
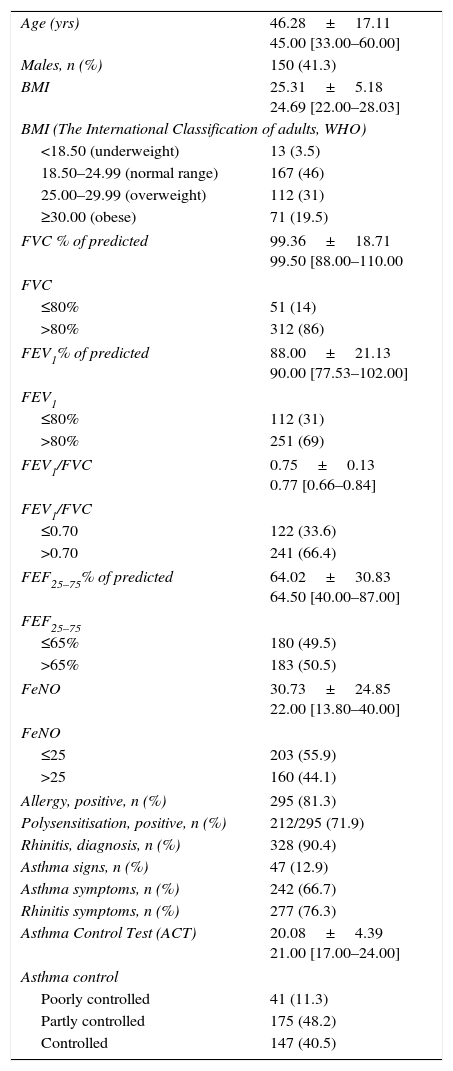
In particular, poorly controlled asthmatics showed the highest FeNO values (median: 42.90, 25th–75th: 19.63–77.15; p<0.001) as shown in Fig. 1, with a probability almost four times greater to have pathological values compared to controlled asthmatic patients (OR: 3.71, 95% CI: 1.74–7.89; p=0.002).
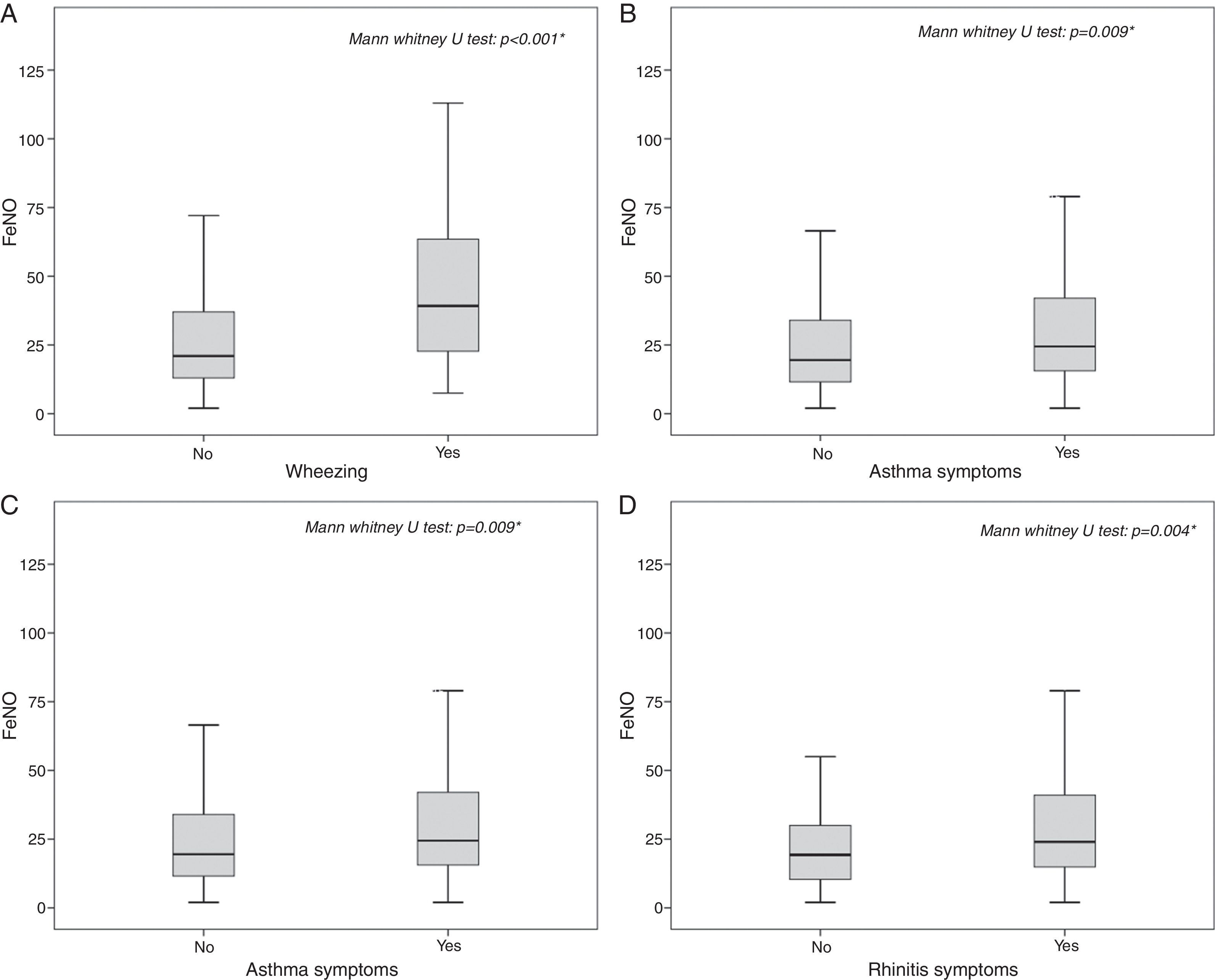
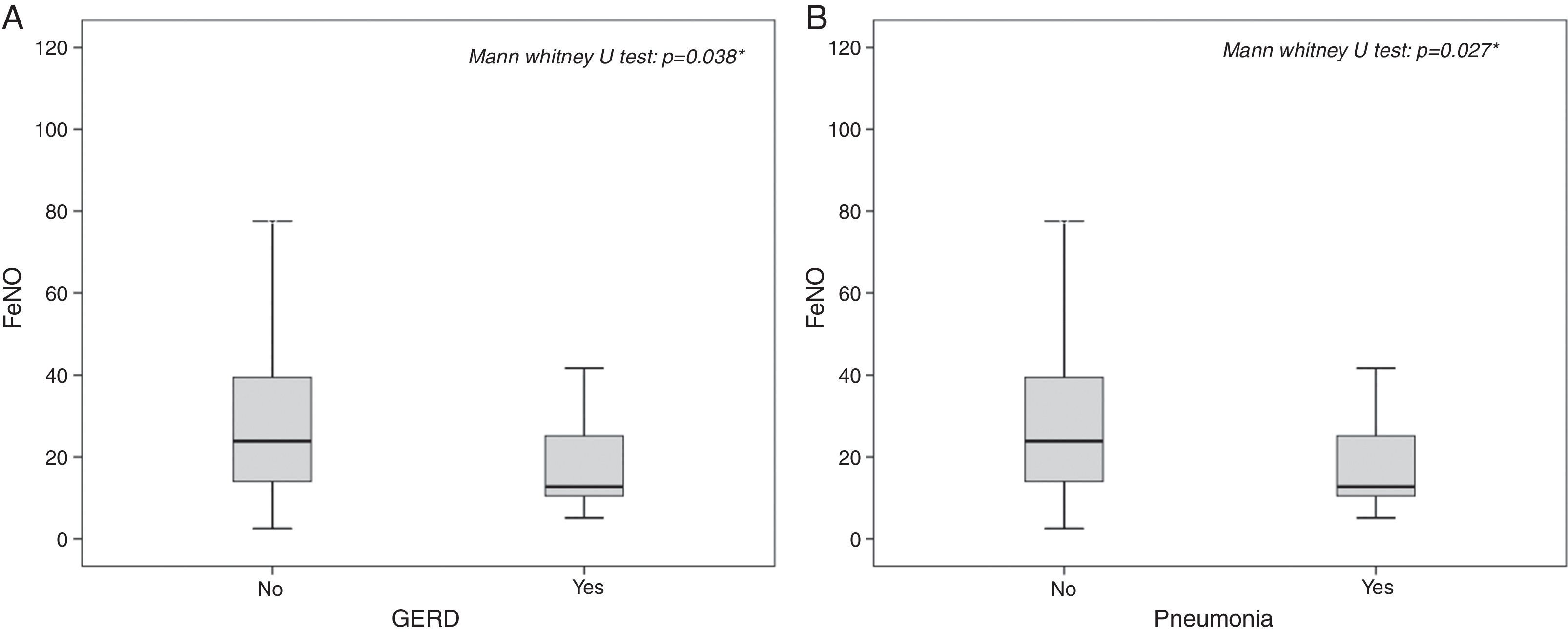
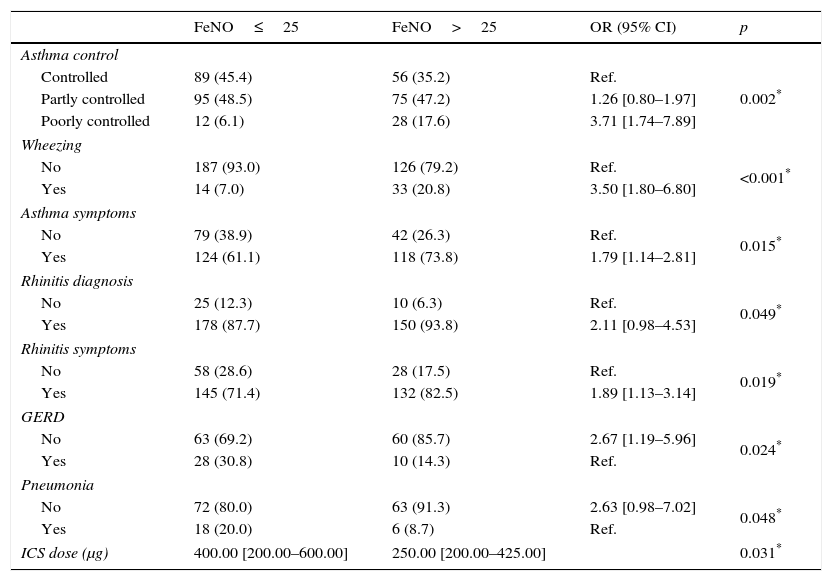
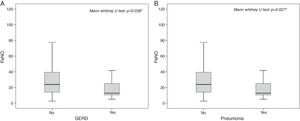
The highest FeNO levels were recorded in subjects with wheezing (p<0.001) (Fig. 2A), asthma symptoms (p=0.002) (Fig. 2B), rhinitis diagnosis (p=0.009) (Fig. 2C), rhinitis symptoms (p=0.006) (Fig. 2D), and in patients without gastro-oesophageal reflux disease (p=0.038) (Fig. 3A) and any history of pneumonia (p=0.027) (Fig. 3A). On the other hand, there was no relevant correlation between ACT scores and FeNO values (r=−0.16).
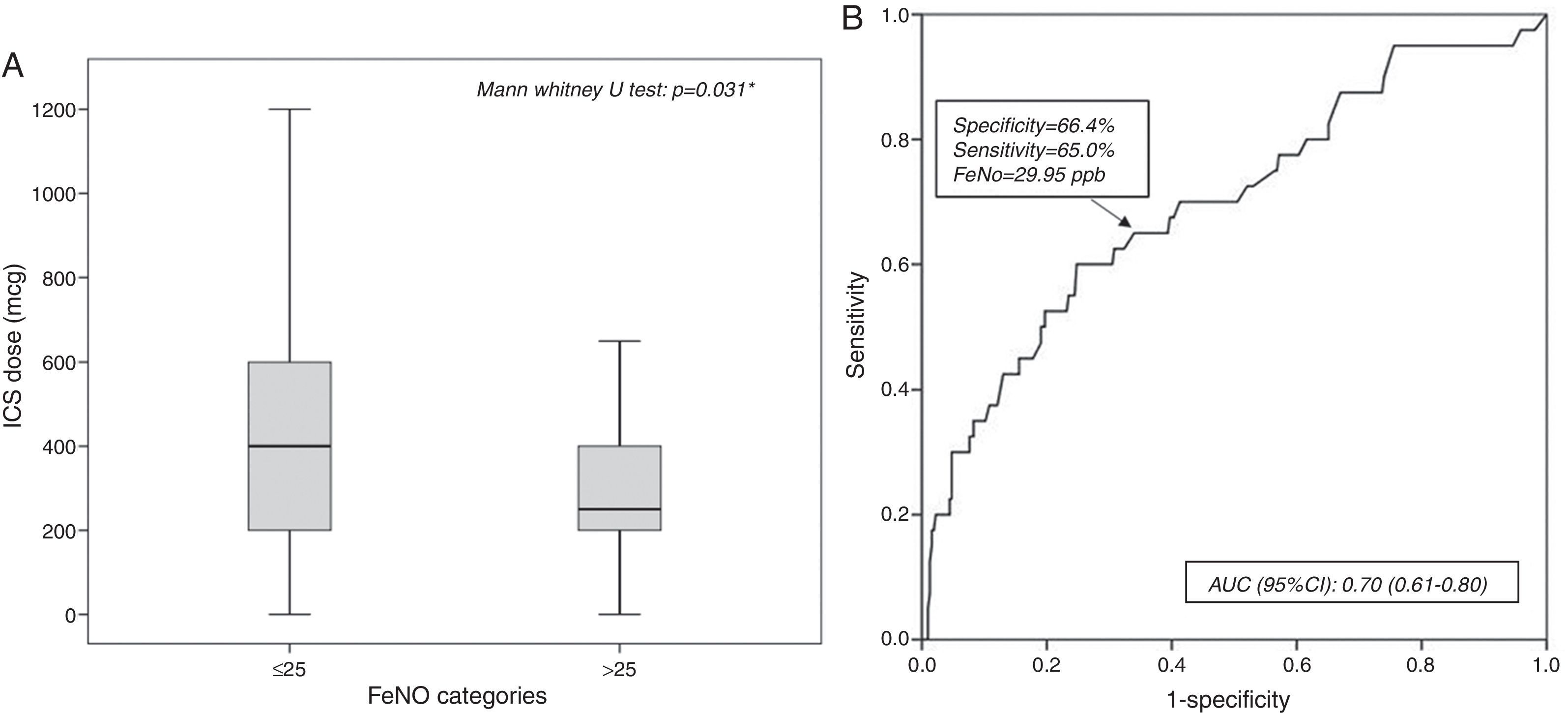
A statistically significant difference in ICS dose (p=0.031) was found between patients with pathological FeNO (median: 250.00μg; 25th–75th: 200.00–425.00) and patients with normal FeNO (400.00μg; 25th–75th: 200.00–600.00) (Fig. 4A).
The probability of pathological FeNO was more than three times higher in wheezing patients compared to patients with negative physical examination (OR: 3.50, 95% CI: 1.80–6.80; p<0.001).
Patients with asthma or rhinitis symptoms had almost double the probability of having a pathological FeNO (for asthma symptoms, OR: 1.79; 95% CI: 1.14–2.81; p=0.015; for rhinitis symptoms, OR: 1.89, 95% CI: 1.13–3.14; p=0.019) and the probability was more than twice if the patient had a diagnosis of rhinitis (OR: 2.11, 95% CI: 0.98–4.53; p=0.050).
In patients either without any gastro-oesophageal reflux complications (OR: 2.67, 95% CI: 1.19–5.96, p=0.024) or without any history of pneumonia (OR: 2.63, 95% CI: 0.98–7.02, p=0.048) the risk to have pathological FeNO values was more than two-fold higher compared to patients with any history of these diseases.
A receiving operator characteristic (ROC) curve was applied to assess how accurate FeNO values were in predicting poorly controlled asthmatic patients. FeNO values can be considered fair predictors for poorly controlled asthma: the area under the ROC curve was 0.70 (95% CI: 0.61–0.80; p<0.001), as reported in Fig. 4B. FeNO value of 29.95ppb (with a sensitivity of 65% and a specificity of 66.4%) was considered the cut-off value to define poorly controlled asthma.
DiscussionFeNO has been considered a surrogate marker of airway inflammation, mainly in allergic patients. FeNO measurement is a simple tool to assess bronchial inflammation and this study aimed to investigate whether FeNO measurement may relate to clinical and/or functional parameters in asthmatics observed in a real life setting.
This study demonstrated that FeNO values were significantly higher in poorly controlled compared to both partly or controlled asthmatics. The highest FeNO values were associated with the presence of asthma signs at clinical examination and asthma symptoms in the past month, rhinitis diagnosis and nasal symptoms in the previous month, and low ICS dose, whereas GERD and pneumonia history were associated with the lowest FeNO values.
The logistic regression highlighted that the presence of impaired FeNO values (>25ppb) was significantly associated with poorly controlled asthma (OR 3.71) as well as asthma signs (OR 3.5) and recent symptoms (OR 1.79), comorbid rhinitis (OR 2.11), and current rhinitis symptoms (OR 1.89). On the other hand, GERD (OR 2.67) and pneumonia history (OR 2.63) were associated with low FeNO values. These findings were confirmed by the ROC curve with fair reliability (AUC 0.70). Therefore, high FeNO values may reflect an asthma state that is not adequately controlled, mainly concerning the presence of asthma signs and symptoms. In addition, the current study underlines the link between rhinitis and asthma: comorbid rhinitis is associated with bronchial inflammation and poorly controlled asthma.
Concerning GERD, this effect on FeNO levels might be explained by pH and airway acidification. Nitrite and nitrate levels in plasma, for example, can reflect the dietary intake rather than NO metabolism in vivo.18 Moreover, NO is also formed enzyme-independently from nitrite under acidic conditions.19 Hunt et al. showed that the airways pH drops dramatically during an acute asthma attack, which facilitates the conversion of nitrite to NO.10 Hence, increased NO concentrations in the exhaled air of asthmatic patients may reflect nitrite conversion rather than NOS activity. Those authors also speculated that therapies directed at normalising airway pH early in the course of an acute exacerbation of asthma will help to prevent the cascade of events that leads to airflow obstruction. In line with the latter observation, we may hypothesise that in our population of asthmatics with GERD the use of pump-proton inhibitors probably influences pH, determining its increase and, as a consequence, a lower production of FeNO. Therefore, our findings are consistent with previous reports underlining these pathophysiological aspects.20–22 Additionally, in patients with inflammatory bowel disease FeNO levels are slightly higher, correlating with disease activity, compared to controls suggesting a systemic involvement of the disease.23
With regard to the relation between eosinophils and pneumonia, it is known that eosinophils may protect from invading pathogens in the lung through mechanisms (e.g. bromination) able to kill bacteria.24,25 In particular, the univalent reduction of oxygen to super oxide anion is the first step in the formation of reactive oxygen species. These compounds can either spontaneously or enzymatically dis-mutate to hydrogen peroxide. Granulocytes contain peroxidases (myeloperoxidase and eosinophil peroxidase) that are able to catalyse the reaction of hydrogen peroxide with halides leading to the formation of hypohalides, e.g., hypochlorous acid.24 Recent gas chromatography–mass spectrometry (GC–MS) studies demonstrated that eosinophils use eosinophil peroxidase (EPO) to generate oxidants in allergen-triggered asthma.25 BrY (3-bromotyrosine), a specific marker of protein modification by reactive brominating species, was markedly enriched in broncho-alveolar lavage (BAL) proteins recovered from asthmatic subjects following exposure to segmental allergen challenge.25 Thus, one chemical pathway used by eosinophils to promote oxidative modification of proteins during asthma is through EPO-generated reactive brominating species. Another potential pathway for oxidative modification of tissues in asthma by eosinophils may involve formation of reactive nitrogen species (RNS). NO production is increased in asthma and NO is a short-lived radical that can be converted into more potent RNS. Thus, we may speculate that high FeNO levels are related to high eosinophilia and, as a consequence, to greater protection against pathogens.
The present findings highlight the close link between bronchial inflammation and asthma control level, as a FeNO value>29.9ppb is associated with poorly controlled asthma. In this regard, several studies provided evidence that FeNO might be a useful biomarker in monitoring of asthma treatment efficacy. Jones et al. reported that FeNO measurement was as useful as induced sputum analysis and BHR in assessing airway inflammation, with the advantage that they are easy to perform, mainly concerning the FeNO usefulness for diagnosing and predicting loss of control in asthmatic patients after corticosteroid withdrawal.26 A longitudinal study in unselected asthmatics showed that a FeNO decrease <40% or increase <30% precluded asthma control optimisation or deterioration, respectively.27 In addition, in ICS-naïve patients, FeNO>35ppb predicted asthma control improvement in response to ICS treatment. However, the overall FeNO ability to reflect asthma control was reduced in patients using high doses of inhaled corticosteroids, whereas FEV1 had little additional value in assessing asthma control.27 The same group reported in a real-world setting study that not only high but also intermediate FeNO levels that are associated with a significant improvement in asthma control after starting ICS treatment.28 Another study showed that the use of FENO to guide anti-inflammatory treatment within primary care significantly reduced the exacerbation rate and improved asthma symptom control without increasing overall inhaled corticosteroid use.29 In a non-blind, pragmatic, cluster-randomised trial in primary care enrolling adults with a doctor's asthma diagnosis, it was concluded that a symptom- plus FeNO-driven strategy reduced asthma medication use and sustained asthma control and quality of life, suggesting that this strategy is preferred for adult asthmatics in primary care.30 Another real life study documented that FeNO was a reliable marker of asthma control in asthmatic patients treated with budesonide/formoterol as acute reliever.31 A retrospective analysis of steroid-naive school-age children with atopic and non-atopic asthma evidenced that FeNO may be used to predict BHR to adenosine monophosphate (AMP) in atopic but not in non-atopic steroid naive asthmatic children.32 In an elderly population the reference ranges have to be changed as the optimal cut-off, for the prediction of asthma, were 30.5ppb for males and 20.5ppb for females.33 Recently, a new algorithm has been developed to compute FeNO at 50ml/s from tidal breathing measurements opening the way to standardised FeNO measurements in preschool children and uncooperative patients.34 Therefore, all these recent studies were consistent with the present report confirming the significant association between FeNO and asthma control grade.
On the contrary, there are also conflicting studies that should suggest caution in interpreting FeNO findings in particular conditions. One study, conducted on 118 patients with a primary care diagnosis of asthma, tested the hypothesis that titrating corticosteroid dose, using the concentration of FeNO, could result in fewer asthma exacerbations (primary endpoint) and more efficient use of corticosteroids, when compared with traditional management.35 The results showed that an asthma treatment strategy based on FeNO measurement did not result in a large reduction in asthma exacerbations or in the total amount of inhaled corticosteroid therapy used over 12 months, when compared with current asthma guidelines.35 A paediatric study reported that common steps in asthma treatment, i.e. inhalation instruction and increasing ICS dose, were both ineffective in reducing FeNO in atopic asthmatic children with elevated FeNO values despite treatment with ICS.36 Thus, the authors concluded that FENO cannot simply be incorporated in current treatment guidelines. Another study performed in young patients with persistent asthma reported that conventional asthma management resulted in good control of symptoms in most participants, whereas FeNO addition as an indicator of asthma control resulted in higher doses of inhaled corticosteroids, without clinically important improvements in symptomatic asthma control.37 A paediatric study assessed daily FeNO 0.05 and/or symptoms tele-monitoring in the management of childhood asthma38 revealing that daily FeNO 0.05 monitoring compared with daily symptom monitoring alone did not added clinical value. Furthermore, a systematic review and meta-analysis reported that tailoring of asthma treatment based on FeNO levels has not been shown to be effective in improving asthma outcomes in children and adults.39 The authors concluded that there was insufficient justification to advocate the routine use of either sputum analysis (due to technical expertise required) or FeNO in everyday clinical practice. A real-life study also reported that in a broad spectrum of asthmatics encountered in clinical practice, sputum eosinophilia and methacholine bronchial hyperresponsiveness, but not FeNO, were associated with uncontrolled asthma.40
However, Turner very recently pointed out that the past studies contributed to the rise and the apparent fall of FeNO as biomarker for asthma, but recent evidence would suggest that FeNO measurement may play a role in the management of childhood asthma, and in particular preventing exacerbations.41 However, it should also consider that in addition to FeNO measurement there are also other alternative non-invasive methods for assessing inflammation in respiratory tract, for example the analysis of exhaled breath condensates with various immunological and biochemical markers, such leukotrienes, cytokines, metallo-proteinases, etc.42,43
The relevance of our study is supported by the consistent number of patients enrolled and by the real life setting. However, possible limitations of this study are the cross-sectional design, the high prevalence of allergic patients, and the absence of comparisons with other inflammation mediators. Another relevant limitation is the lack of checking for the presence of chronic rhinosinusitis (CRS) with or without nasal polyposis, which may mimic classical rhinitis. These disorders have been described as being important determinants of asthma control and FeNO as their presence, in particular nasal polyps, is strictly related to worse asthma control and very high levels of FeNO, independently of concomitant asthma.44 These findings are also consistent with the current ones, as the current study reported the absence of a relationship between ACT scores and FeNO. Nasal polyps, but not FeNO, have been reported to be the only significant determinant of poor asthma control.45 In addition, nasal polyps and asthma have been shown to be independent determinants of high FeNO values in patients with chronic rhinosinusitis. Moreover, there is now evidence that one of the most severe phenotypes of severe asthma is the refractory eosinophilic one, which is described to be very often associated with nasal polyposis, increasing the evidence that nasal polyps are important determinants of a more severe asthma. Therefore, further studies should be conducted to consider this relevant issue.
In conclusion, the current study provides evidence that high FeNO is related to poorly controlled asthma and that relevant clinical features are associated with impaired FeNO values. These outcomes may suggest assessing FeNO in all naïve asthmatics in order to identify those with high levels (phenotyping), in whom the monitoring of FeNO may be useful to obtain clinically-related information for practical asthma management.
Ethical disclosuresProtection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Patients’ data protectionThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Conflict of interestAll authors contributed to the paper and none of them has any conflict of interest.