The factors – including asthma and rhinoconjunctivitis – which influence FeNO values in a general population of school children have been studied in order to know to what extent the variability of those values can be explained.
MethodsFeNO was measured in a population of 240 school children aged 6–12 years by means of a Niox-Mino™ device in a standardised way. Parents filled in an ISAAC-validated questionnaire of symptoms and environmental factors. Diagnoses were checked against clinical records. Height and weight were measured. A multivariate regression analysis including all variables in the questionnaire was performed, which was followed by two Xi stepwise tests in order to build a predictive model which included the main variables influencing FeNO values.
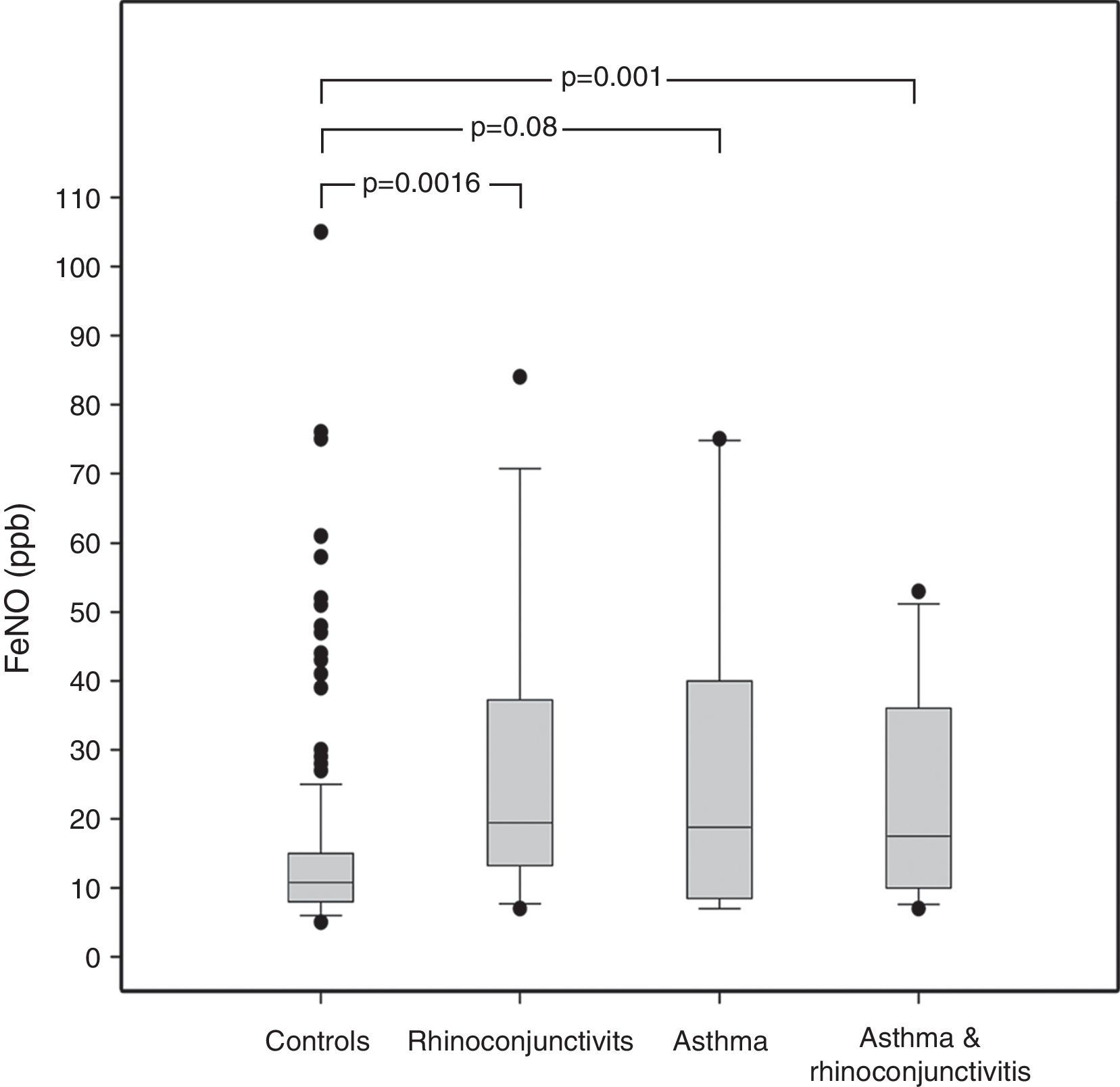
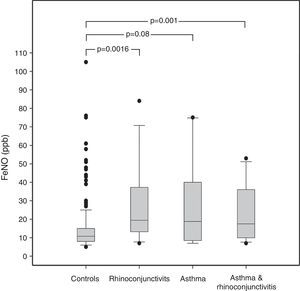
ResultsAmong the 240 children, 10 suffered from asthma, 16 from rhinoconjunctivitis and 15 from both conditions. FeNO values (GM±GSD) in children with rhinoconjunctivitis (19.61±1.20ppb), with asthma (18.62±1.32ppb), and with both conditions (17.62±1.19ppb) tended to be significantly higher than control children (11.42±1.04ppb), p=0.0016, p=0.08 and p=0.01, respectively. The different predictive models were able to explain only 20–27% of FeNO variability.
ConclusionsThe proportion of FeNO inter-individual variability which can be explained by individual (including suffering from asthma or rhinoconjunctivitis), family, and environmental factors is very low (20–27%). This could have implications on the usefulness of FeNO as a diagnostic tool in asthma.
Asthma consists of chronic hyper-responsiveness and variable inflammatory obstruction, affecting the airways. This chronic condition leads to airway wall remodelling, which appears to be associated with persistent airway inflammation, initially without clinical evidence of airway obstruction. Abnormally thickened airways may be the mechanism underlying both bronchial hyper-responsiveness and fixed loss of respiratory function.1
Fractional exhaled Nitric Oxide (FeNO) has been reported as a non-invasive airway inflammation marker in asthma. In fact, FeNO, measured in the general population, has shown higher levels in children with symptoms of asthma and atopy.2 However, the interpretation of FeNO levels should be taken with caution as their predictive value is unknown. Although higher FeNO values are associated to asthma, the presence of other atopic conditions such as allergic rhinoconjunctivitis or atopic dermatitis may confound this association. Furthermore, FeNO was found to be increased in asthmatic children and in children with atopic disorders, regardless of the presence of respiratory symptoms.2,3 However, not only asthma or atopy, but also other factors such as age, gender, height, body mass index, tobacco smoking, parental asthma/allergy, and allergen sensitisation have been shown to exert an influence on FeNO values.4–7
The aim of the present study is to determine whether other still non-studied environmental circumstances might influence FeNO values in school children; and to know to what extent individual (including atopy defined by positive prick-test, asthma and/or rhinoconjunctivitis), family, and environmental factors could explain FeNO inter-individual variability.
MethodsStudy populationIn order to avoid variability in the study due to non-Caucasian ethnicity, all children aged 6–12 in a public primary school with the lowest rate of immigrants in the area (2%) were invited to participate (n=466). The ISAAC validated questionnaire was distributed in the school to the children's parents, with an explanatory letter attached, which included information on how to fill in the questionnaire and about the FeNO measurement method. The completed questionnaire was brought back and given to the teacher in each class.
EthicsAn informed consent form was included with each questionnaire. The Ethics Committee o the “Los Arcos del Mar Menor” University hospital approved the study.
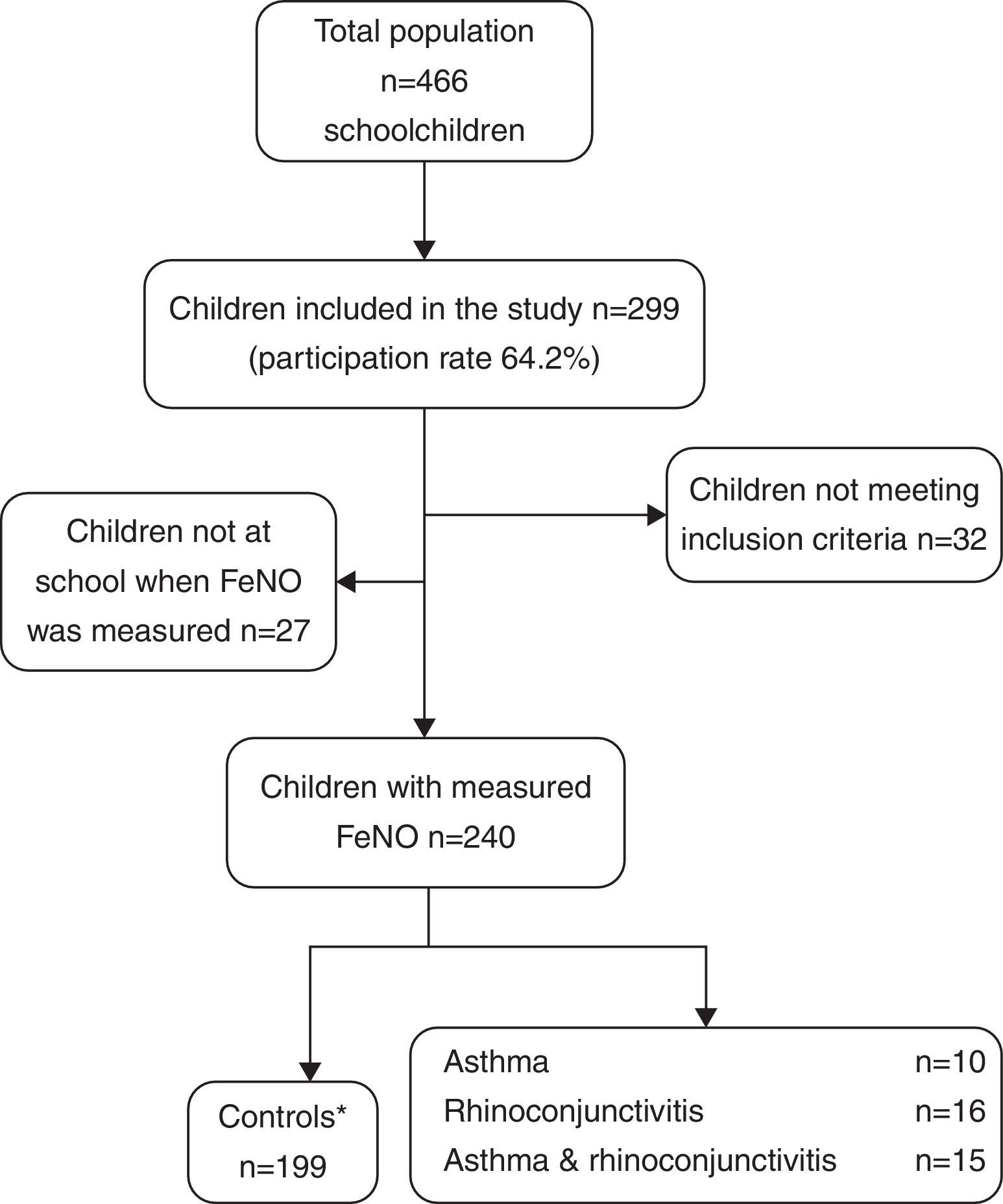
Selection of childrenIn order to build the definite dataset, the following criteria were used:
- •
Inclusion criteria: Boys and girls, aged 6–12 (both inclusive) with a correctly completed questionnaire, and fewer than seven attempts for a correct FeNO measurement.
- •
Exclusion criteria: Discordant diagnoses between questionnaire and clinical record, being currently on inhaled corticosteroids, incorrect/incomplete questionnaire, more than seven attempts for FeNO measure performance, non-Caucasian ethnicity.
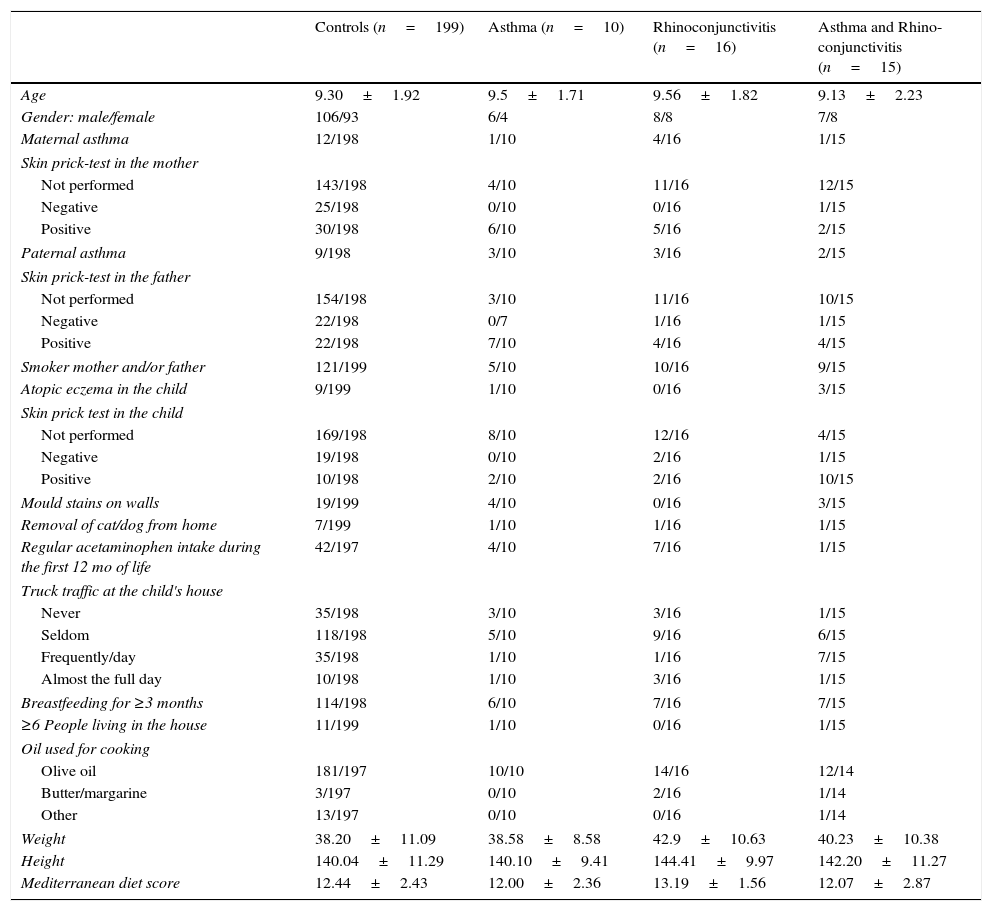
Data were collected using the ISAAC phase III questionnaire (core and environmental questionnaires) (http://isaac.auckland.ac.nz). The environmental questions include a food frequency (per week) questionnaire (never or occasionally, 1–2 times, 3 or more); parental history of asthma, rhinitis or atopic dermatitis (Yes/No); smoking habits of father and/or mother (Yes/No); number of people living in the family house; months of exclusive breastfeeding; preterm birth (Yes/No); presence of mould stains on the household walls (Yes/No); truck traffic in the street where the family house is located (never/seldom/frequently during the day/almost the full day); pet removal from home (Yes/No); skin prick test performed (Yes/No; if Yes, Positive/Negative) in the children and parents (which was checked against the clinical record when performed); regular acetaminophen intake for fever during the first year of life (Yes/No); and oil used for cooking. Symptoms of asthma were defined as a positive answer to the question: “Has your child had wheezing or chest whistling in the chest in the past 12 months?” Similarly, symptoms of rhinoconjunctivitis were defined as a positive answer to both questions: “Has your child had itchy nose or sneezing, not related to a cold in the previous 12 months?” and “Have these nose problems come together with itchy or red eyes?” Atopy eczema was defined as a positive response to: “Has your child had an itchy rash located in the folds of the elbows/behind the knees/in front of the ankles/under the buttocks/around the neck, ears or eyes in the last 12 months?” The Mediterranean diet score was calculated according to the method previously used.8 All factors included in the study are shown in Table 1.
Demographic characteristics of the four groups of children in which the study population was divided prior to calculating any predictive model (proportions and mean±SD).
| Controls (n=199) | Asthma (n=10) | Rhinoconjunctivitis (n=16) | Asthma and Rhino-conjunctivitis (n=15) | |
|---|---|---|---|---|
| Age | 9.30±1.92 | 9.5±1.71 | 9.56±1.82 | 9.13±2.23 |
| Gender: male/female | 106/93 | 6/4 | 8/8 | 7/8 |
| Maternal asthma | 12/198 | 1/10 | 4/16 | 1/15 |
| Skin prick-test in the mother | ||||
| Not performed | 143/198 | 4/10 | 11/16 | 12/15 |
| Negative | 25/198 | 0/10 | 0/16 | 1/15 |
| Positive | 30/198 | 6/10 | 5/16 | 2/15 |
| Paternal asthma | 9/198 | 3/10 | 3/16 | 2/15 |
| Skin prick-test in the father | ||||
| Not performed | 154/198 | 3/10 | 11/16 | 10/15 |
| Negative | 22/198 | 0/7 | 1/16 | 1/15 |
| Positive | 22/198 | 7/10 | 4/16 | 4/15 |
| Smoker mother and/or father | 121/199 | 5/10 | 10/16 | 9/15 |
| Atopic eczema in the child | 9/199 | 1/10 | 0/16 | 3/15 |
| Skin prick test in the child | ||||
| Not performed | 169/198 | 8/10 | 12/16 | 4/15 |
| Negative | 19/198 | 0/10 | 2/16 | 1/15 |
| Positive | 10/198 | 2/10 | 2/16 | 10/15 |
| Mould stains on walls | 19/199 | 4/10 | 0/16 | 3/15 |
| Removal of cat/dog from home | 7/199 | 1/10 | 1/16 | 1/15 |
| Regular acetaminophen intake during the first 12 mo of life | 42/197 | 4/10 | 7/16 | 1/15 |
| Truck traffic at the child's house | ||||
| Never | 35/198 | 3/10 | 3/16 | 1/15 |
| Seldom | 118/198 | 5/10 | 9/16 | 6/15 |
| Frequently/day | 35/198 | 1/10 | 1/16 | 7/15 |
| Almost the full day | 10/198 | 1/10 | 3/16 | 1/15 |
| Breastfeeding for ≥3 months | 114/198 | 6/10 | 7/16 | 7/15 |
| ≥6 People living in the house | 11/199 | 1/10 | 0/16 | 1/15 |
| Oil used for cooking | ||||
| Olive oil | 181/197 | 10/10 | 14/16 | 12/14 |
| Butter/margarine | 3/197 | 0/10 | 2/16 | 1/14 |
| Other | 13/197 | 0/10 | 0/16 | 1/14 |
| Weight | 38.20±11.09 | 38.58±8.58 | 42.9±10.63 | 40.23±10.38 |
| Height | 140.04±11.29 | 140.10±9.41 | 144.41±9.97 | 142.20±11.27 |
| Mediterranean diet score | 12.44±2.43 | 12.00±2.36 | 13.19±1.56 | 12.07±2.87 |
Height was measured without shoes using a Seca™ stadiometer (graduated scale with mobile head support) with an error range of ±1cm. At least two measures were taken, relocating the child between each measure. Subsequently, weight was measured without shoes and wearing light clothes, using a Seca™ scale, calibrated daily, with an error range of ±1kg.
FeNo testFeNO tests were performed at the school setting, using the portable Niox-Mino™ device (Aerocrine, Solna, Sweden) as follows: the patients made an initial expiration and then they made a deep inspiration to inhale NO-free air through the disposable filter up to total lung capacity. Immediately afterwards, they breathed the air out through the filter into the device at an exhalation rate of 50ml/s, which was controlled by a light and acoustic sensor. NO environment was considered to be <5ppb and measurements were always performed in the same room and at the same time of the day. Children have a lower lung capacity than adults, which could hinder them from exhaling correctly for more than 6s. Thus, an exhalation period of 6s has been validated for the portable Niox-Mino™ device.9,10 The 6-s exhalation time was used in all tests. Following American Thoracic Society and European Thoracic Society (ATS/ETS) recommendations,11 children did not perform more than seven attempts for FeNO measurement, and two consecutive values were registered.
Statistical analysisFeNO values fitted in a log-normal distribution; thus the geometric mean (GM) and the 95% confidence interval (95% CI) were reported for every group of children. Kruskal–Wallis statistic was used to test the difference between each group (controls, asthma, rhinoconjunctivitis, or asthma and rhinoconjunctivitis). Statistical calculations were carried out by means of STATA SE v10 (Stata Corp., College Station, TX, USA).
All four groups of children were pooled together and FeNO values transformed to their logarithm. A first multivariate linear regression analysis was performed. All variables were included in this model (Model 1). Akaine Information Criteria (AIC) and Bayesian Information Criteria (BIC) were calculated to check the model's quality based on all variables. A link test was also performed to verify that no more variables were required to explain the model. Finally, two Xi stepwise tests, with pr(0.2) and pr(0.05), were applied to extract the main variables that defined the model. Thus, a second and a third model (Model 2 and Model 3) were obtained, after recalculating the multivariate linear regressions with the variables extracted from the first model, including AIC and BIC; and the corresponding link test to ascertain that it was correctly calculated and that no more variables than those required had been removed from the previous model.
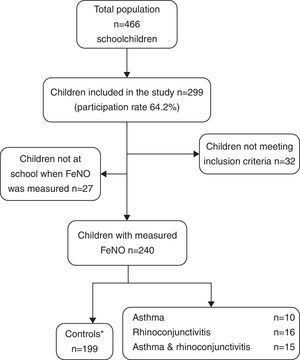
ResultsThe demographic characteristics of the study population are shown in Table 1. Among the 240 children included in the study (Fig. 1), 10 suffered from asthma, 16 from rhinoconjunctivitis and 15 from both conditions. FeNO values (GM and 95% CI) in children with rhinoconjunctivitis (19.6ppb; 95% CI 13.3–28.9ppb), with asthma (18.6ppb; 95% CI 9.8–35.2ppb), and with both conditions (17.2ppb; 95% CI 12.1–25.5ppb) tended to be significantly higher than those without these conditions (11.4ppb; 95% CI 10.5–12.4ppb), p=0.0016, p=0.08 and p=0.01, respectively (Fig. 2).
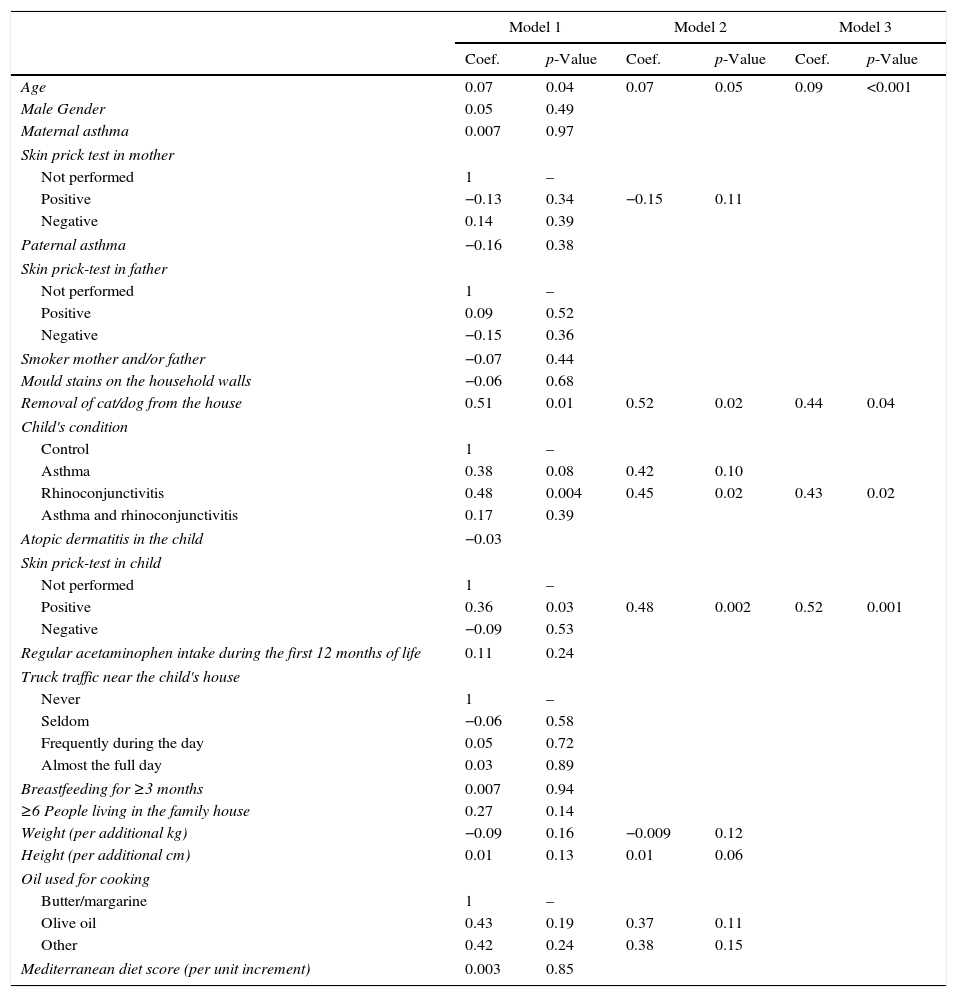
The multivariate linear regression model which was applied to estimate the predictive power of each variable included in our questionnaire to build Model 1 (Table 2), yielded a R2 value indicating that the percentage of FeNO variability explained with this model was 27%. The link test showed that no more variables were required to explain FeNO variability in this model (p<0.0001, R2=0.27). The Xi stepwise test pr(0.2), performed to extract the main variables explaining that 27% of variability yielded Model 2, which included the following eight variables: age; positive skin prick test in the mother; cat/dog removal from the house; asthma or rhinoconjunctivitis in the child; positive skin prick test in the child; weight; height; and using oil vs. margarine/butter for cooking. Model 2 still explained 23% of FeNO variability. According to AIC and BIC Model 2 was better than Model 1 (AIC 4.20 vs. 4.46; BIC 4.58 vs. 5.46). Finally, a new link test revealed that no more variables than required were removed from Model 1 (p<0.001, R2=0.23).
Multivariate regression models: influence of each variable (expressed as a coefficient) and the 95% confidence intervals for the three models.
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| Coef. | p-Value | Coef. | p-Value | Coef. | p-Value | |
| Age | 0.07 | 0.04 | 0.07 | 0.05 | 0.09 | <0.001 |
| Male Gender | 0.05 | 0.49 | ||||
| Maternal asthma | 0.007 | 0.97 | ||||
| Skin prick test in mother | ||||||
| Not performed | 1 | – | ||||
| Positive | −0.13 | 0.34 | −0.15 | 0.11 | ||
| Negative | 0.14 | 0.39 | ||||
| Paternal asthma | −0.16 | 0.38 | ||||
| Skin prick-test in father | ||||||
| Not performed | 1 | – | ||||
| Positive | 0.09 | 0.52 | ||||
| Negative | −0.15 | 0.36 | ||||
| Smoker mother and/or father | −0.07 | 0.44 | ||||
| Mould stains on the household walls | −0.06 | 0.68 | ||||
| Removal of cat/dog from the house | 0.51 | 0.01 | 0.52 | 0.02 | 0.44 | 0.04 |
| Child's condition | ||||||
| Control | 1 | – | ||||
| Asthma | 0.38 | 0.08 | 0.42 | 0.10 | ||
| Rhinoconjunctivitis | 0.48 | 0.004 | 0.45 | 0.02 | 0.43 | 0.02 |
| Asthma and rhinoconjunctivitis | 0.17 | 0.39 | ||||
| Atopic dermatitis in the child | −0.03 | |||||
| Skin prick-test in child | ||||||
| Not performed | 1 | – | ||||
| Positive | 0.36 | 0.03 | 0.48 | 0.002 | 0.52 | 0.001 |
| Negative | −0.09 | 0.53 | ||||
| Regular acetaminophen intake during the first 12 months of life | 0.11 | 0.24 | ||||
| Truck traffic near the child's house | ||||||
| Never | 1 | – | ||||
| Seldom | −0.06 | 0.58 | ||||
| Frequently during the day | 0.05 | 0.72 | ||||
| Almost the full day | 0.03 | 0.89 | ||||
| Breastfeeding for ≥3 months | 0.007 | 0.94 | ||||
| ≥6 People living in the family house | 0.27 | 0.14 | ||||
| Weight (per additional kg) | −0.09 | 0.16 | −0.009 | 0.12 | ||
| Height (per additional cm) | 0.01 | 0.13 | 0.01 | 0.06 | ||
| Oil used for cooking | ||||||
| Butter/margarine | 1 | – | ||||
| Olive oil | 0.43 | 0.19 | 0.37 | 0.11 | ||
| Other | 0.42 | 0.24 | 0.38 | 0.15 | ||
| Mediterranean diet score (per unit increment) | 0.003 | 0.85 | ||||
Model 1: R2=0.27; p<0.001. Model 2: R2=0.23; p<0.001. Model 3: R2=0.20; p<0.001.
The same procedure was used to build Model 3 from Model 2, but using Xi stepwise test pr(0.05). Model 3 included only four variables, i.e. age, removal of cat/dog from the house, rhinoconjunctivitis and positive skin prick test in the child. Model 3 could explain 20% of FeNO variability. In this case, the link test also indicated that the model was correctly specified (p<0.001, R2=0.20). AIC and BIC showed that Model 3 was slightly better than Model 2 (AIC 4.19 vs. 4.20; BIC 4.36 vs. 4.58) (Table 2).
DiscussionThe results of the present study show that FeNO levels tended to be significantly higher in children with asthma and/or rhinoconjunctivitis, which is consistent with previous studies.5,7,12
A FeNO cut-off value to predict asthma has not been definitely established in children, and the attempts to find it have yielded considerable variability in their diagnostic accuracy.12–17 The low agreement for FeNO cut-off points and diagnostic accuracies is probably explained by the different methods used to measure FeNO, as well as the by the heterogeneity of the study populations. Furthermore, this difficulty to find a specific cut-off point may be further increased by a high inter-individual variability even among healthy children in the control groups. However, the present study was not designed to test FeNO measurements as a diagnostic tool, but to determine factors predicting FeNO values in a general population.
Factors associated to FeNO valuesSeveral factors are associated to FeNO levels, such as those shown in Table 2. Gender has been considered as a factor influencing FeNO levels.7,18 However, other studies are in agreement with the results of the present study, which did not find a significant association between FeNO and gender.4 Atopic dermatitis has been previously considered a condition influencing FeNO levels,3 although our regression model has rejected its relevance. According to one study,7 a family history of allergic diseases, which is definitely associated to a higher risk of suffering from an atopic condition in the offspring does not seem to influence FeNO values when no allergic disease is present in the child. However, a positive skin prick test in the mother (but not asthma or rhinoconjunctivitis) is the only factor in the family history that influences FeNO levels in the present study. Pet allergy has been related with the increase of FeNO levels,7 and as much as one third of the FeNO variability (higher than in the present study) has been said to be predicted by allergic sensitisation to indoor allergens.4 Nevertheless, this was found in a very specific population of children at risk of allergy and asthma who might have increased baseline values and in whom only a very powerful influencing factor, such as sensitisation to a perennial allergen, might have shown any influence on FeNO variability. Yet our results show that removing cat and/or dog from the house in a general population, which is probably a marker of allergy to those pets within the family, is also associated to higher levels of FeNO.
Other factors which were significantly associated to FeNO in our study were height and weight and thus being responsible for some inter-individual variability. This association has already been shown in previous studies6,12 but not in all of them.18 However, those last studies did not analyse the variability factor. The use of olive oil for cooking was significantly associated to lower FeNO levels in the present study. There is some evidence supporting Mediterranean diet (including the use of olive oil as a main source of polyunsaturated fatty acids) as a protective factor for asthma, at least in the Mediterranean basin.19 Thus, it is plausible that lower FeNO levels are associated with frequent olive oil consumption. Age was one of the most influential factors in FeNO values: this association was also been shown previously.4–7,12
Our results show that asthma and rhinoconjunctivitis are conditions significantly associated to FeNO. Surprisingly, comorbidity was not found to be associated to FeNO. We do not have a clear explanation for this, beyond the fact that the number of children with any of those conditions was very low.
Variability and prediction of FeNOSee et al.12 had previously built a prediction FeNO model, in which ethnicity, height, self-reported rhinoconjunctivitis and household smoke exposure were the main factors capable of explaining only 10.3% of FeNO variability in a US general population of children aged 6–11. However, they used questionnaire-based diagnosis and did not include prick test. Contrary to their model, smoker mother and/or father did not significantly explain FeNO variability in our Model 1, thus it was not included in further Models 2 and 3. Our predicting models agree in including height and rhinoconjunctivitis as the four main factors explaining FeNO variability. However, their model did not include skin prick-test to define atopy, as already mentioned, which is one of the four variables included in our last predicting Model 3. Another limitation of their study is that asthma and/or rhinoconjunctivitis diagnosis was self-reported, rather than a questionnaire-validated diagnosis. Despite this possible classification bias, our models agree in including rhinoconjunctivitis as a main factor predicting FeNO variability. Both the absence of prick-test and the lack of validated diagnosis asthma/rhinoconjunctivitis could explain why our model practically duplicates the power of their predicting model (10.3% vs. 20%).
Considering that our best model (Model 1), which included 20 variables, only explained 27% of variability then we are still far away from a model that reaches a decent prediction power. It is interesting that a model with only four variables (age, rhinoconjunctivitis, positive skin prick test and removal of cat and/or dog from the house) was still capable of explaining 20% of variability. These results lead us to consider that FeNO measurement may not be a good tool to clearly diagnose asthma and/or rhinoconjunctivitis in the general population of schoolchildren. This is indeed independent of the fact that FeNO levels tended to be significantly higher in children with asthma and/or rhinoconjunctivitis. The need to use four variables to explain just 20% of FeNO inter-individual variability is in high contrast with only two factors (age and height) explaining 50% of FEV1 inter-individual variability in healthy subjects.20
It seems that FeNO variability needs to be better explained in order to be considered a useful tool to establish the difference between asthmatic and non-asthmatic children in the general population. The sources of the unexplained variability are difficult to establish; however, at least two of them could be hypothesised. Firstly, there is genetic variability: the NO pathway system genes are quite diverse and different polymorphisms cause variability in healthy children; this variability being modified by the presence of asthma.21 Additionally certain polymorphisms in other genes have been recently shown to be associated to FeNO values (albeit explaining less than 1% of its variability).22 Secondly, and as some authors have suggested,23,24 FeNO might be associated to changes in the lung surface. It is probably a dual influence: quality and quantity of the airway epithelium. The fact that FeNO increases with age both in healthy children, as shown previously23,24 and in the present study (significant and positive coefficients in all regression models – Table 2), and in asthmatic ones25 is probably related – at least in part – to the increase in lung volume and airway surface area.24 Additionally, increasing age and/or recurrent immunological stimulation could bring about changes in the airway NO diffusion, or on the stimulation of the inducible nitric oxide synthase.25,26 It should also be considered that factors of different nature acting on the airways in the recent (infections, air pollution, tobacco smoke, food rich in nitrites, etc.)27–29 or in the remote (preterm birth, bronchopulmonary dysplasia, etc.)30 past might have disrupted the normal development and maturation of the airway surface and thus explain FeNO variability to a certain extent. The situation becomes even more complicated when recent data show that FeNO is associated to the different asthma phenotypes in children, persistent wheezers having significantly higher values that transient wheezers or controls.31,32
LimitationsIt could be argued that the main weakness of the present study is the low number of children with asthma and/or rhinoconjunctivitis, however it should be kept in mind that it was performed in a general population in order to study variability. In spite of the expected low number of children with asthma/rhinoconjunctivitis, the multivariate analysis found that these conditions were influencing FeNO significantly and the mean values were also significantly higher as compared to controls. Furthermore, this factor was a very significant one which had to be included in all regression models. The fact that cases detected by the ISAAC questionnaire were checked against the clinical record, make classification bias quite unlikely for those cases. However, it would have been possible that children who had had asthma and/or rhinoconjunctivitis-like symptoms in the first years of life remained undetected by the questionnaire due to recall bias and thus not checked against the clinical records, and consequently included among the healthy controls. A similar rationale could be applied to children with very mild current symptoms who might remain sub-clinical. This would have tended to lower the difference between cases and controls (which was still highly significant) but would probably not have modified the effects of the studied factors on the inter-individual variability of FeNO values. The main strength of the present study lies in its relatively high sample size, which allowed us to perform a multivariate regression including up to 20 factors. Other strengths would lie in its epidemiological nature and on the amount of epidemiological factors analysed.
ConclusionsAlthough FeNO variability is highly associated with several factors, mainly age and allergic markers, the proportion of FeNO inter-individual variability which can be explained by individual (including suffering from asthma or rhinoconjunctivitis), family and environmental factors is quite low (20–27%). This could have implications on the usefulness of FeNO as a diagnostic tool in asthma.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Confidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Conflict of interestThe authors declare no conflict of interest.
We would like to thank Mr. Anthony Carlson for his help with the editing of the English language.