Atopic dermatitis (AD) is most common in the first year of life. The aim of this study was to determine the prevalence of and risk factors for AD in a birth cohort of infants from southeast Turkey.
MethodsAdana Paediatric Allergy Research (ADAPAR) birth cohort study was derived from 1377 infants who were born in Cukurova University, Medical Hospital, Adana, Turkey between February 2010 and February 2011. At birth, a physical examination was performed, cord blood samples were taken, and the mother completed a baseline questionnaire that provided data on gestational conditions, family history of allergic diseases and environmental exposures. Follow-up visits scheduled at 3, 6, and 12 months included an infant physical examination and an extended questionnaire. Skin prick test was performed and food-specific IgE levels were measured at 6 and 12 months. Atopic dermatitis was diagnosed based on confirmatory examination by a physician.
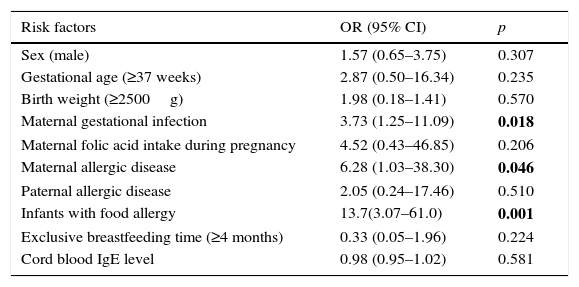
ResultsOf the 1377 infants enrolled, 59 (4.3%) were diagnosed with AD as of 12 months. Maternal allergic disease (ORs 6.28, 95% CI 1.03–38.30; p=0.046), maternal infection during gestation (ORs 3.73, 95% CI 1.25–11.09; p=0.018), and presence of food allergy (ORs 13.7, 95% CI 3.07–61.0; p=0.001) were identified as risk factors for AD. Breastfeeding and cord blood IgE levels were not identified as risk factors.
ConclusionsIn this cohort we found prevalence of AD as 4.3% during the first year of life. Positive family history of atopic diseases, prenatal infections and presence of food allergy are the risk factors for early presentation of AD.
Atopic dermatitis (AD), also known as “atopic eczema” and “eczema,” is the most common chronic and recurrent skin disorder of childhood and is being reported with increasing frequency worldwide. The aetiopathogenesis of this condition is complex, and disruption of the skin barrier, impaired natural immune responses, and overreaction to allergens and microbial agents are considered to play roles. Environmental factors related to industrial development and abnormal immune response to these factors early in intrauterine life are also thought to contribute.1
Research has demonstrated that foetal immunoglobulin E (IgE) production begins at 11 weeks of gestation and that sensitisation to allergens may even begin in utero.2 Many investigations have been conducted since the first report suggested that high IgE levels in cord blood (CB) could predict future development of allergic disorders; however, the relationship between CB-IgE level and childhood atopic diseases remains controversial.3 Studies have identified numerous factors that affect CB-IgE but it is still not clear exactly how these levels are influenced by the foetal environment, including maternal, paternal, placenta, and foetal characteristics.4
In many countries, birth cohort studies have been carried out to determine the prevalence of and risk factors for AD. These investigations have examined comparable age groups, and the reported prevalence of AD has ranged from 10% to 28%.1,5 To date, no such study has been done in Turkey and relatively few data are available from southern Europe.1 The aim of the Adana Paediatric Allergy and Risk Factors (ADAPAR) birth cohort study was to establish the prevalence of AD and identify associated risk factors by following infants in the city of Adana (southeast Turkey) from birth until 1 year of age.
Materials and methodsStudy designThe investigation was a population-based, single-centre, birth cohort study with unselected participants. In total 1475 infants born at Çukurova University Medical Hospital in Adana, Turkey were recruited as potential participants between February 2010 and February 2011. The study protocol was approved by the university's Human Research Ethics Committee. Written informed consent was obtained from the parents of each infant who was enrolled.
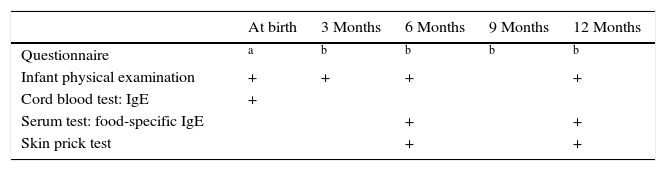
Multiple evaluations were done at birth and during follow-up (Table 1). At birth, each infant was clinically examined by a physician and CB sampling was performed. Two types of questionnaires related to the infants were administered during the study: baseline (completed by the mother at birth) and follow-up (multiple time points; Table 1). The baseline questionnaire provided information on the mother's gestational conditions (i.e. pre-existing diseases, nutritional supplements, medications, tobacco use), family history of allergic diseases (e.g., asthma, allergic rhinitis, food allergy, and AD), potential allergens in the household (e.g., smoking, pets, mould), and the family's demographic data. Each neonate's birth data (gender, weight, gestational age at birth) were collected from medical reports.
Follow-up visits were scheduled for 3, 6, and 12 months of age. At each visit, the same physician examined the infant and the mother completed an extended follow-up questionnaire. This instrument gathered data on breastfeeding and the infants’ diet, as well as infant nutritional supplements, infections, medications, vaccinations, smoking in the house, any signs and symptoms of allergic disease that the mother had observed in her baby. Telephone interviews were conducted when the infants were 9 months of age and the same extended questionnaire was administered at that time. As well, at the 6- and 12-month visits, each infant was scheduled to undergo a skin prick test (SPT) and blood testing for quantification of food-specific (FS) IgE. In every case where a follow-up examination was missed, the mother was contacted and completed the same questionnaire by telephone.
The interview at 12 months of age was completed by the mothers of 1377 (93.3%) of the infants, and this defined the participants who were enrolled in the study.
Cord blood sampling and IgE testingFor each infant, immediately after birth, the umbilical cord was cleansed with a sterile gauze swab and 5mL of CB were aspirated from the umbilical vein of the placenta into a syringe. Each sample was immediately centrifuged at 3000rpm for 15min, and serum was separated and stored −30°C until it was analysed. Levels of CB-IgE were determined using the ImmunoCAP® Specific IgE test (Unicap, Phadia, Uppsala, Sweden) and results were expressed as kU/L. To ensure results were not confounded by sample contamination with maternal blood, on the same day that the IgE testing was done, each CB serum sample was tested for IgA (expressed as mg/dL) at the Çukurova University Biochemistry Laboratory. In any case where the IgA result was ≥11mg/dL, the infant's CB-IgE result was excluded.
Food-specific IgE testingInitially, each infant's serum was screened for the six most common food allergens using an ImmunoCAP® kit. If this test was positive, then the serum was analysed for specific IgE antibodies for cow's milk, hen's eggs, soy, wheat, fish, and peanuts. Results ≥0.35kU/L were accepted as positive.
Skin prick testAn SPT was performed using a commercially available extracts of major inhalant allergens (Allergopharma, Germany): tree mixture (alder, hazel, poplar, elm, sallow), mould mixture (Alternaria alternata, Cladosporium herparum, Fusarium moniliforme), pollen mixture (grass, barley, oat, rye, wheat, velvet, orchard, rye, timothy, blue grass, and meadow fescue), Dermatophagoides pteronyssinus and farinae, and food allergens (milk, egg, wheat, peanut, and banana). The SPTs were performed using standard methods,6 and the result for each allergen was defined as positive if the mean wheal size was >3mm larger than the negative control.
Diagnosis of atopic dermatitis and food allergyGiven that the infants were in the first year of life, AD was diagnosed using the simplified criteria established by Williams.7 Information on AD outcomes was obtained from the questionnaires asking the history of confirmed AD by any paediatrician/dermatologist in our clinic or another institution.
Infants with history of skin reaction or respiratory and/or gastrointestinal system reaction after a specific food intake, those with FS-IgE ≥0.35kU/L, and those with a positive SPT were further evaluated with an elimination diet and were then offered an open food challenge (OFC).8 Food allergy was defined as a positive OFC test.
Statistical analysisData were analysed using SPSS for Windows v.15.0. Findings in the groups with and without AD were statistically compared. Relationships between AD in infancy and various maternal, environmental and perinatal risk factors were assessed using the Pearson χ2 test. The Mann–Whitney U test was used to compare the groups CB-IgE concentrations. Multiple logistic regression was used to analyse the effects of factors that were identified as statistically significant or were considered clinically important relative to AD. Results were presented as odds ratios (ORs) with 95% confidence intervals (95% CI). A p value below 0.05 was considered significant.
ResultsTest results and group comparisonsOf the 1475 potential participants, 1377 infants (93.3%; 732 boys and 645 girls) were ultimately enrolled. The main reasons for declining to participate were a family move, serious illness and poor parental understanding.
At the end of the 1-year study period, 920 infants had undergone at least one SPT and/or FS IgE determination. By this stage, 59 (4.3%) of the 1377 infants had been diagnosed with AD.
The mean CB-IgE level for the 1377 infants was 28.9±19.2kU/L and the median was 25.0kU/L (range, 13–49kU/L). The mean CB-IgE levels for the groups of infants with and without AD were not significantly different (26.95±2.54kU/L vs. 29.15±1.9kU/L, respectively; p>0.05).
Of the 59 infants diagnosed with AD, food allergy was defined in 12 infants (20.3%) to a total of 14 foods based on OFC results. Egg white allergy was determined in nine infants and cow's milk allergy in five infants. None of the infants with AD was sensitive to inhalant allergens.
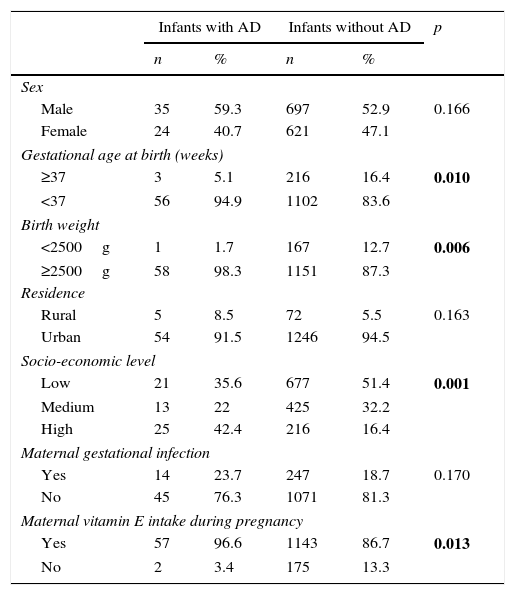
Table 2 summarises comparisons of demographic and questionnaire findings for the groups with and without AD. Frequencies of premature birth and low birth weight were significantly lower for the infants with AD (p=0.010 and 0.006, respectively). The proportions of mothers who had received folic acid and vitamin E during pregnancy were higher for the group with AD (p=0.026 and 0.013, respectively). Frequencies of paternal allergic disease, maternal allergic disease, and presence of food allergy in the infant were also significantly higher for the group with AD (p=0.004, 0.001 and 0.001, respectively). There were no significant differences between the two groups with respect to infants’ sex distribution, maternal smoking during pregnancy, household exposures (smoking, humidity/mould, pets), exclusive breastfeeding time, or total duration of breastfeeding (all p>0.05).
Comparison of demographic and questionnaire findings in the groups with and without atopic dermatitis (AD).
| Infants with AD | Infants without AD | p | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Sex | |||||
| Male | 35 | 59.3 | 697 | 52.9 | 0.166 |
| Female | 24 | 40.7 | 621 | 47.1 | |
| Gestational age at birth (weeks) | |||||
| ≥37 | 3 | 5.1 | 216 | 16.4 | 0.010 |
| <37 | 56 | 94.9 | 1102 | 83.6 | |
| Birth weight | |||||
| <2500g | 1 | 1.7 | 167 | 12.7 | 0.006 |
| ≥2500g | 58 | 98.3 | 1151 | 87.3 | |
| Residence | |||||
| Rural | 5 | 8.5 | 72 | 5.5 | 0.163 |
| Urban | 54 | 91.5 | 1246 | 94.5 | |
| Socio-economic level | |||||
| Low | 21 | 35.6 | 677 | 51.4 | 0.001 |
| Medium | 13 | 22 | 425 | 32.2 | |
| High | 25 | 42.4 | 216 | 16.4 | |
| Maternal gestational infection | |||||
| Yes | 14 | 23.7 | 247 | 18.7 | 0.170 |
| No | 45 | 76.3 | 1071 | 81.3 | |
| Maternal vitamin E intake during pregnancy | |||||
| Yes | 57 | 96.6 | 1143 | 86.7 | 0.013 |
| No | 2 | 3.4 | 175 | 13.3 | |
| Maternal folate intake during pregnancy | |||||
| Yes | 55 | 93.2 | 1104 | 83.5 | 0.026 |
| No | 4 | 6.8 | 214 | 16.2 | |
| Maternal smoking during pregnancy | |||||
| Yes | 4 | 6.8 | 162 | 12.3 | 0.102 |
| No | 55 | 93.2 | 1156 | 87.7 | |
| Smoking in the home | |||||
| Yes | 25 | 42.4 | 629 | 47.7 | 0.211 |
| No | 34 | 57.6 | 689 | 52.3 | |
| Humidity/mould in the home | |||||
| Yes | 7 | 11.9 | 138 | 10.5 | 0.368 |
| No | 52 | 88.1 | 1178 | 89.5 | |
| Pets in the home | |||||
| Yes | 3 | 5.1 | 103 | 7.8 | 0.221 |
| No | 56 | 94.9 | 1214 | 92.2 | |
| Maternal allergic disease | |||||
| Yes | 15 | 25.4 | 134 | 10.2 | 0.001 |
| No | 44 | 74.6 | 1184 | 89.8 | |
| Paternal allergic disease | |||||
| Yes | 6 | 10.2 | 46 | 3.5 | 0.004 |
| No | 53 | 89.8 | 1272 | 96.5 | |
| Infants with food allergy | |||||
| Yes | 12 | 20.3 | 21 | 1.6 | 0.001 |
| No | 47 | 79.7 | 1297 | 98.4 | |
| Exclusive breastfeeding time | |||||
| <4 months | 19 | 32.2 | 414 | 31.4 | 0.449 |
| ≥4 months | 40 | 67.8 | 904 | 68.6 | |
| Total duration of breastfeeding | |||||
| ≤6 months | 9 | 15.3 | 138 | 10.5 | 0.253 |
| >6 months | 48 | 81.4 | 1136 | 86.2 | |
| No breast milk fed | 2 | 3.4 | 44 | 3.3 | |
The results regarding effects of potential risk factors for childhood AD are presented in Table 3. Food allergy in the infant (OR 13.7, 95% CI3.07–61.0), maternal allergic disease (OR 6.28, 95%CI 1.03–38.30), and maternal infection during gestation (e.g., upper airway infection, pneumonia, amnionitis, or urinary tract infection) (OR 3.73, 95%CI 1.25–11.09) were identified as risk factors for the development of AD.
Risk factors for development of atopic dermatitis based on results of multivariate logistic regression analysis.
| Risk factors | OR (95% CI) | p |
|---|---|---|
| Sex (male) | 1.57 (0.65–3.75) | 0.307 |
| Gestational age (≥37 weeks) | 2.87 (0.50–16.34) | 0.235 |
| Birth weight (≥2500g) | 1.98 (0.18–1.41) | 0.570 |
| Maternal gestational infection | 3.73 (1.25–11.09) | 0.018 |
| Maternal folic acid intake during pregnancy | 4.52 (0.43–46.85) | 0.206 |
| Maternal allergic disease | 6.28 (1.03–38.30) | 0.046 |
| Paternal allergic disease | 2.05 (0.24–17.46) | 0.510 |
| Infants with food allergy | 13.7(3.07–61.0) | 0.001 |
| Exclusive breastfeeding time (≥4 months) | 0.33 (0.05–1.96) | 0.224 |
| Cord blood IgE level | 0.98 (0.95–1.02) | 0.581 |
IgE, immunoglobulin E.
The ADAPAR birth cohort study is the first study in Turkey that has investigated the prevalence of AD and associated risk factors in infants. At the end of the first year of life, 4.3% of our sample (59 of 1377 infants from southeast Turkey) had been diagnosed with AD, a prevalence lower than reported in other birth cohort studies from European countries.9,10 Our study required that diagnosis of AD be confirmed by a clinician, whereas other researchers have frequently based this diagnosis on data from questionnaires alone or on recorded medical data alone. Confirmation by a physician is more accurate than other methods of diagnosing AD; therefore, this difference is important. Notably, some studies on infants from northern European countries have documented higher prevalences of physician diagnosed AD than we observed.1,5
The relationship between AD and food allergy is well known. The majority of cases of AD diagnosed during infancy are associated with food allergens.11 Most cross-sectional studies report 30–40% frequencies of allergen sensitivity among children with AD.11,12 In our study, this frequency was found to be as low as 20.3%. It is suggested that one possible reason for this result is a positive OFC was required to diagnose food allergy in our study. Similar to our investigation, Johnke et al. conducted a birth cohort study of infants diagnosed with AD that followed 562 babies for the first 18 months of life.13 They reported 26% with food allergies based on SPT, and found that this proportion dropped to 11.5% based on OFC. No inhalants allergen sensitivity was determined on any of the infants at the end of 12 months in our study. While other cohort studies in literature have reported inhalants allergen sensitivity in the same age group, it is a very interesting point that indoor allergens like cat and house dust are the ones mainly seen in these.14 Additionally, pet ownership has been linked with allergic sensitisation and development of AD.15 These studies have reported that more than 50% of the infants’ homes had pets (cats, dogs), in our study this number was a clearly low rate of 5.6%. The majority of the infants, who were taken into the study at the same time, were living in city centres, the infants with AD had high a socioeconomic level and therefore their exposure to animals was quite low. Another reason is that the inhalants allergen specific IgE level was not measured; only the inhalants allergen sensitivity was evaluated with SPT. Furthermore, the reporting of different ratios of allergen sensitivities from different countries brings to mind that, many different unknown mechanisms might be interacting with each other and playing a common role in the AD aetiology. In this study, we present the first data of a prospective birth cohort study, which evaluates the possible influence of nutritional, environmental and genetic factors in our country. The paucity of data for the prevalence of AD and associated risk factors in Turkey and neighbouring countries complicates the interpretation of our results. Further follow-up of the children should clarify these factors’ contribution of AD.
Extensive research has indicated that hereditary factors are important in the development of AD. We observed that infants born to mothers with a history of allergic disease were at slightly greater risk for AD, which is consistent with previous findings.16,17 Research has identified that predisposing genes of the maternal line are more important than those of the paternal line in the development of allergic disorders. Transfer of maternal antibodies and cytokines to the infant via the placenta or through breastfeeding is known to potentially affect the development of atopic disorders.17 Moreover, it has been reported that history of combined maternal and paternal allergic disease is a greater risk factor than a single parent affected for AD.9
There was no significant difference between infants of AD and without AD with respect to maternal infection rates. Surprisingly, maternal infection during gestation was identified as a risk factor for development of AD according to multiple logistic regression analysis. A large number of exposures were assessed (ranging from pregnancy and foetal related, environmental, genetic, dietary factors, as well as household level). The combination of several factors could affect the results of the logistic model in this study. Therefore, it is difficult to confirm a causal link between maternal gestational infection and AD even in our large prospective studies. Similarly, another study that evaluated the effects of prenatal factors on development of atopic disorders revealed that infections during gestation increase an infant's risk for allergic diseases, such as AD, in early childhood.18 One explanation for this is that, during an infection, microorganisms and toxins affect uterine and placental function, and this alters the immunological development of the foetus.19 It is being reported that this situation causes abnormal immune activation and response and thereby playing a role in the development of allergic diseases. It is thought that maternal antibiotic use due to infection in the intrauterine period is causing allergic response by changing the intestinal microflora.20,21 Lately it has been shown that, with the basal Th2 response developed in rats which were given kanamycin treatment, there is IgE and IgG1 increment in the circulation and reduction in IFNγ production.22 In our study, we did not collect data of antibiotic use during pregnancy. Therefore, we did not examine any correlation between antibiotic use during pregnancy and atopic dermatitis. Therefore, we are restricted from making any further comments.
Many studies have explored the association between the development of AD and elevated CB-IgE levels. Birth cohort studies demonstrated a positive correlation between elevated CB-IgE level and AD.23,24 Still, the link between CB-IgE level and childhood atopic disease remains controversial. Other birth cohort studies have reported no association between elevated CB-IgE level and development of AD in infants,25 and the results of our study are consistent with these findings.
Frequencies of premature birth and low birth weight were significantly lower for the infants with AD in our study. A retrospective study by Trønnes et al. demonstrated higher risk of AD among full-term infants than among preterm infants.26 Linneberg et al. found that risk for AD increased as birth weight increased27 and Hikino et al. observed that risk for AD was lower among infants with low birth weight.28 The activity of adipokines released from adipose tissue during intrauterine life has been implicated in impairment of tolerance mechanisms. Research suggests that AD is associated with high levels of IL-6, leptin, and tumour necrosis factor-alpha in obese patients.29
We found that infants with AD had significantly higher proportions of mothers who had taken vitamin E and folic acid, respectively. Various studies that have investigated the role of free oxygen radicals and vitamin E levels in the development of allergic disorders have reported controversial results.30 Nwaru et al. observed no relationship between vitamin E levels at one year of age and development of AD in a cohort study.9 Recent epidemiological studies have reported an association between folic acid supplementation during pregnancy and increased risk of allergic disease.31,32 It is known that the dominant T helper (Th) 2 profile in the foetal immune system is associated with the development of allergic diseases. It has also been demonstrated that a methyl donor, such as folic acid, is associated with altered lymphocyte development and may alter the Th1/Th2 balance in favour of Th2.33
Our study has potential limitations. First, only 920 (67%) of the infants enrolled had undergone physical examination, SPT and/or FS IgE determination by 1 year of age. In other words, for 33% of the infants, information of confirmed AD was obtained from the questionnaires completed by the parents. This is a high proportion. In these patients AD was diagnosed by any clinician; therefore, this should not have resulted in misclassification. Second, environmental exposures (with the exception of pregnancy-related factors) were self-reported and not clinically investigated. For example, mothers were only questioned regarding their vitamin E and folate supplementation during pregnancy; we did not actually measure serum levels in mothers and/or infants. This needs to be considered regarding conclusions about the relationship between AD and maternal intake of folate and vitamin E.
Overall, we observed a 4.3% prevalence of AD at 1 year of age in this birth cohort, and our study indicates that the presence of food allergy in an infant, maternal allergic disease, and maternal infection during gestation are associated with AD in 1-year-old children from southeast Turkey. We observed no relationship between development of AD and CB-IgE level, the environmental factors investigated, or breastfeeding in our cohort. Longer-duration follow-up of the ADAPAR birth cohort is planned, and this could help identify possible risk factors associated with onset of AD in older age groups of children.
Ethical disclosuresProtection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals fort his investigation.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and\ or subjects mentioned in the article. The author for correspondence is in possession of this document.
FundingThis study was supported by a research Grant (TF2010LTP17) from Çukurova University.
Conflict of interestThe authors have no conflict of interest to declare.