Gelsolin is an actin-binding protein with several cellular functions including anti-apoptosis and is reported to have an anti-inflammatory effect. Apoptosis of keratinocytes has been implicated as a key mechanism of atopic dermatitis (AD).
ObjectiveWe aimed to determine plasma gelsolin (pGSN) levels in children with atopic dermatitis (AD).
MethodThe diagnosis of AD was made according to Hanifin and Rajka criteria. The disease severity was scored by objective SCORAD index by the same allergist. Skin prick testing (SPT), total IgE levels, and eosinophil counts were analyzed. The pGSN levels were determined using ELISA technique.
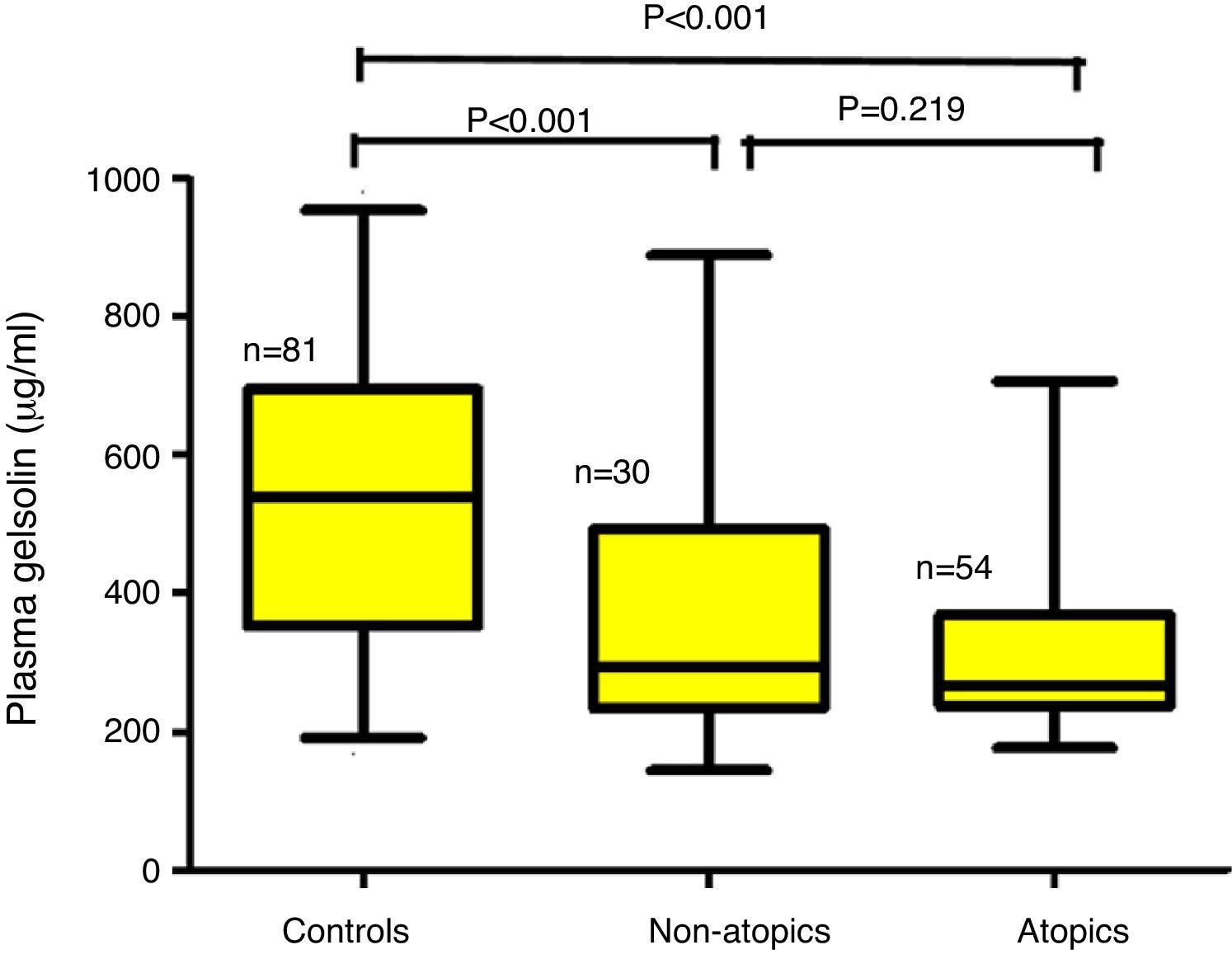
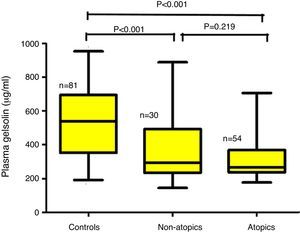
ResultsChildren aged between 0.5 and 3.0 years were included in the study. The children with AD (AD; n=84) were analyzed in two groups according to the presence (AD+/Atopy+; n=54) or absence of SPT positivity (AD+/Atopy−; n=30). The comparisons were made with a healthy control group matched for age and sex (n=81). The median (interquartile range) of pGSN levels in AD+/A+, AD+/A− and control groups were 267μg/ml (236–368), 293 (240–498) and 547 (361–695), respectively (p<0.001). The difference between the control group and AD sub-groups remained significant after Bonferroni correction (p<0.001). Correlation analysis failed to reach significance with the disease severity total IgE levels and eosinophil counts.
ConclusionThis is the first study investigating the association of pGSN levels with AD and disease severity. pGSN levels decreased in AD. These findings suggest that gelsolin may have a role in the disease process in AD patients.
Atopic dermatitis (AD) is a common, chronic inflammatory skin disease that affects more than 10% of all children.1 Acute AD lesions exhibit typical histopathological characteristics such as epidermal spongiosis that sometimes leads to vesicle formation and a perivascular inflammatory infiltration in the dermis.1 Particularly, it is believed that keratinocyte apoptosis mediated by Fas/Fas ligand molecular interactions and subsequent caspase activation are involved in the pathogenesis of AD.2
The actin cytoskeleton plays a role in the regulation of apoptosis through interplay with mitochondria. The assembly and disassembly of actin filaments in addition to the organization of these filaments into functional high order networks are regulated by several actin-binding proteins including gelsolin.3–5 Gelsolin is a molecule that contains six domains, G1–G6 starting from the N terminus. There is a Ca2+-binding site within each domain. Three binding sites play an important role in F-actin severing and nucleation; namely monomer actin binding sites in G1, G4-6 and highest actin binding site in G2-3. F-actin severing by gelsolin involves first the binding of G2 to F-actin. This disrupts the actin–actin hydrophobic bond interactions between two actin monomers, which result in severing. Thereafter, gelsolin remains attached to the barbed ends of actin filaments and acts as a cap. Thus, a new actin polymerization from the barbed end uncapping of gelsolin is required.3–5 Gelsolin is involved in main cellular processes including cell motility, proliferation, and apoptosis. It may enhance or inhibit apoptosis processes based on the nature of the pathological conditions, the identity of the cell types, and involved tissues.5–8 It regulates the apoptotic pathway via substrate of caspase-3, -6, -9, and/or -8. Caspase-3 cleaves gelsolin between residues Asp352 and Gly 353 to generate fragments with molecular masses of 39kDa (N-terminal half) and 41kDa (C-terminal half). The resultant N-terminal gelsolin fragment not only severs actin in a Ca21 independent manner but is also pro-apoptotic.5–8 In contrast, full-length gelsolin, especially human gelsolin (hGSN), the C-terminal half of gelsolin, and gelsolin complexed with phosphatidylinositol 4,5-bisphosphate are generally anti-apoptotic.5,7
Gelsolin has a major role in localizing inflammation and preventing systemic escape of pro-inflammatory lipids.9–11 Consistent with these proposed functions, it was proven that declining pGSN level is associated with various acute clinical conditions.4,12–15 Gelsolin levels have rarely been studied in patients with chronic inflammatory disorders16 and allergic diseases.17–19
In the present study, we aimed to determine pGSN levels in atopic dermatitis and to identify whether there is a relationship between pGSN and disease severity.
MethodsStudy populationThis is a prospective, randomized case–control study conducted in the Department of Pediatric Allergy and Immunology outpatient clinic of a tertiary center between January and March, 2014. The major inclusion criterion was the presence of current AD in children aged 6–36 months (patient group). The diagnosis of AD was made according to Hanifin and Rajka criteria.20 The disease severity was scored by objective SCORAD index by the same allergist.21 Patients who have additional chronic skin disease and any other disorder were excluded from the study. The children within the control group were brought to the Outpatient Clinic of the General Pediatrics Department for a routine healthy child follow-up (control group). The demographic, clinical, and laboratory data of the patients were recorded. Total IgE levels were measured by using Immuno-CAP technique (Pharmacia & Upjohn, Uppsala, Sweden) and eosinophil count was performed by using Siemens Acvia 2120 analyser. All of the children's blood samples were drawn into ethylenediaminetetraacetic acid (EDTA) tubes, and then plasma was removed from the blood, and plasma samples were stored at −80°C until analyzed.
Ethical disclosuresThis study was approved by the Ethics Committee of Erciyes University (18.04.2014/2014-259) and was conducted in accordance with the World Medical Association and the Helsinki Declaration (http://www.wma.net/s/ethicsunit/helsinki.htm). All parents gave written informed consent before participation.
Measurements of plasma gelsolin concentrations in plasmaThe research plasma samples were tested by pGSN (human) enzyme-linked immunosorbent assay (ELISA) kit (product number SK00384-01, Aviscera Bioscience, USA). PGSN of patients were measured by a solid phase Immunoassay method which was designed to measure human soluble gelsolin in serum and plasma. This assay employed the quantitative sandwich enzyme immunoassay technique. Results were expressed as μg/ml.
Statistical analysisThe parameters of the study population (pGSN, total IgE, and eosinophil count) were not normally distributed and were given with median as well as interquartile ranges. Spearman correlation analysis was used to test the relationship between pGSN, IgE, eosinophil count and objective SCORAD scores. Groups were compared by Kruskall–Wallis test with Bonferroni correction, SPSS 15.0 software program (SPSS, Inc., Chicago, IL, USA) was used for all the statistical analysis. A p-value <0.05 was considered statistically significant.
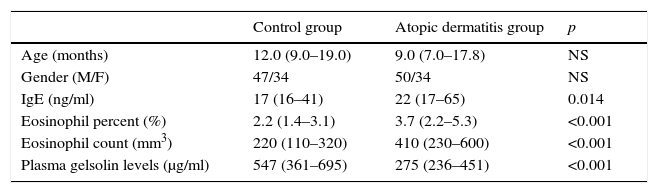
ResultsOf the 165 children included, 84 had AD while 81 were employed as healthy controls. The age range was 6–36 months with a median (interquartile) age of 10 months, M/F=97/68. The characteristics of the study population are presented in Table 1.
Characteristics of the study population.
| Control group | Atopic dermatitis group | p | |
|---|---|---|---|
| Age (months) | 12.0 (9.0–19.0) | 9.0 (7.0–17.8) | NS |
| Gender (M/F) | 47/34 | 50/34 | NS |
| IgE (ng/ml) | 17 (16–41) | 22 (17–65) | 0.014 |
| Eosinophil percent (%) | 2.2 (1.4–3.1) | 3.7 (2.2–5.3) | <0.001 |
| Eosinophil count (mm3) | 220 (110–320) | 410 (230–600) | <0.001 |
| Plasma gelsolin levels (μg/ml) | 547 (361–695) | 275 (236–451) | <0.001 |
NS: non-significant, Ig: Immunglobulin.
The median (interquartile range) of pGSN levels in AD+/A+, AD+/A− and control groups were 267μg/ml (236–368), 293 (240–498) and 547 (361–695), respectively (p<0.001) (Fig. 1). The difference between AD+/A+, AD+/A− and the control group remained significant after Bonferroni correction (p<0.001). We could not find any statistically significant difference in patients with AD+/A+ and AD+/A−. There were no significant correlations between pGSN levels and, total IgE levels, eosinophil counts, and disease severity.
DiscussionTo the best of our knowledge, this is the first study to investigate the role of pGSN levels in children with AD. Our results demonstrate that the pGSN levels were decreased in children with AD when compared to those in the control group. However, no significant differences were observed between patients with AD+/A+ and AD+/A−. There was a significant difference between AD and control groups regardless of the presence of atopy. No marked effect of atopy on gelsolin was observed. This result suggests that the role of pGSN in AD disease process could be associated with different mechanisms other than the IgE-mediated type 1 hypersensitivity reactions.pGSN scavenges and severs actin filaments released from cells during inflammation and injury in order to prevent rises in blood viscosity and possible toxicity of insoluble F-actin filaments.22 Some diseases and tissue injuries of various organs including major trauma, idiopathic lung injury, sepsis, adult respiratory distress syndrome, acute liver injury, allogeneic stem cell transplantation, burns, and sepsis leads to prolonged reductions in pGSN.4,12–15,23,24 pGSN and a peptide based on gelsolin residues bind lipopolysaccharide (LPS) and lipoteichoic acid (LTA).9,25 These molecules are bacterial wall components of Gram-negative and Gram-positive bacteria.9 It also binds other mediators, including lysophosphatidic acid (LPA), β-amyloid peptide, and platelet-activating factor (PAF).9,11 It is thought that pGSN exerts its anti-inflammatory effect by inactivating these molecules through binding them. In addition to decreased levels in acute inflammatory conditions, it was shown that pGSN levels were also decreased in chronic inflammatory conditions as well. Osborn et al. demonstrated that circulating pGSN levels were significantly lower in patients with rheumatoid arthritis compared with healthy controls.16 They also showed that intra-articular pGSN levels were significantly lower than in the paired plasma samples.16 Mucus plugging resulting in infection and inflammation leads to airway wall destruction in patients with cystic fibrosis (CF). Sputum samples from CF patients were shown to have filamentous actin. Human plasma gelsolin, a protein that severs actin filaments, led to rapid decrease in the viscosity of CF sputum samples in vitro.26 This finding suggested an important role for gelsolin in the barrier function of airway surface liquid (ASL) barrier function, indicating that exogenous gelsolin might be considered in the treatment of diseases characterized by chronic inflammation with significant levels of filamentous actin release.
The natural course of AD is a chronic inflammatory process progressing with acute exacerbations. We found low levels of pGSN in AD patients. Our findings about the plasma levels of gelsolin were in agreement with the studies summarized above. Gelsolin levels have rarely been studied in patients with allergic diseases. Kawamoto et al. provided the first evidence indicating that the gelsolin family molecule Der f 16 is a new class of house dust mite allergen.18 Because the gelsolin superfamily is common and highly conserved in multicellular organisms, it could be expected that this protein family may serve as a pan-allergen with IgE cross-reactivity.18 Some authors have proposed that IL-4 could modulate the proteins secreted into the airway surface liquid (ASL) to recover mucociliary clearance or could raise antimicrobial defence in asthma.19 In human bronchial epithelial cell cultures, it was shown that gelsolin is the most abundant protein that breaks down actin filaments.19 In another study, Janciauskiene et al. demonstrated that some patients receiving immunotherapy have higher pGSN levels compared to those without immunotherapy but pGSN levels were similar to those of healthy controls.17
T cell-mediated, Fas-induced keratinocyte apoptosis plays a main pathogenetic role in the AD.2 Janciauskiene et al. reported that allergic rhinitis patients without immunotherapy had lower levels of anti-apoptotic protein, namely gelsolin, indicating a direct link between plasma levels of gelsolin and soluble forms of Fas and Fas-L, markers of apoptosis and systemic inflammation.17 The finding of lower pGSN levels in the present study suggested to us that anti-apoptotic effects of pGSN are more important than the apoptotic effects in patients with AD.
This study has several limitations. First, it includes local practice patterns, a small number of children with AD from a single institution and it may not reflect the real life conditions in population based cohorts. Secondly, the population of the study was restricted to children with AD, so we could not be sure whether these findings were generalized to the adult patients. Thirdly, the gelsolin levels in the skin tissue, which is the target organ, were not detected in the study, therefore, the correlation of gelsolin between site of injury and circulation was still unclear. Because of these limitations, our study results may not be applicable to other institutions or other clinical settings.
These findings suggest that gelsolin may have an important role in the disease process of AD; thus, its depletion may reflect ongoing inflammation and apoptosis. Further larger-scale studies are needed to repeat our results.
Conflict of interestThe authors declared no conflicts of interest.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.